Cloning and Expression of Functional Reteplase in Escherichia coli TOP10
-
Khodabakhsh, Fatemeh
-
Department of Pharmaceutical Biotechnology, Isfahan Pharmaceutical Sciences Research Centre, School of Pharmacy, Isfahan University of Medical Sciences, Isfahan, Iran
-
Dehghani, Zohreh
-
Department of Pharmaceutical Biotechnology, Isfahan Pharmaceutical Sciences Research Centre, School of Pharmacy, Isfahan University of Medical Sciences, Isfahan, Iran
-
Zia, Mohammad Farid
-
Department of Pharmaceutical Biotechnology, Isfahan Pharmaceutical Sciences Research Centre, School of Pharmacy, Isfahan University of Medical Sciences, Isfahan, Iran
-
Rabbani, Mohammad
-
Department of Pharmaceutical Biotechnology, Isfahan Pharmaceutical Sciences Research Centre, School of Pharmacy, Isfahan University of Medical Sciences, Isfahan, Iran
-
 Mir Mohammad Sadeghi, Hamid
Department of Pharmaceutical Biotechnology, Isfahan Pharmaceutical Sciences Research Centre, School of Pharmacy, Isfahan University of Medical Sciences, Isfahan, Iran, Tel: +98 311 7922616; Email: H_sadeghi@pharm.mui.ac.ir
Mir Mohammad Sadeghi, Hamid
Department of Pharmaceutical Biotechnology, Isfahan Pharmaceutical Sciences Research Centre, School of Pharmacy, Isfahan University of Medical Sciences, Isfahan, Iran, Tel: +98 311 7922616; Email: H_sadeghi@pharm.mui.ac.ir
-
Department of Pharmaceutical Biotechnology, Isfahan Pharmaceutical Sciences Research Centre, School of Pharmacy, Isfahan University of Medical Sciences, Isfahan, Iran
Abstract: Background: Production of tissue Plasminogen Activator protein (t-PA) in prokaryotes systems has many problems such as the lack of active protein production, multiple purification steps, and renaturation process which has been shown to be costly and time-consuming. Methods: In this study, reteplase which is the nonglycosylated active domain of t-PA was used to transform TOP10 Escherichia coli (E. coli) bacteria to resolve some of the above mentioned problems. Reteplase cDNA was ligated into pBAD/gIII plasmid which allowed secretion of this protein into the periplasmic space and would allow the correct formation of disulfide bonds in protein structure. The presence of reteplase cDNA in pBAD/gIII plasmid was confirmed by restriction digestion and sequencing. After induction of the expression of this protein by adding 0.0002% L-Arabinose to the medium, the proteins in periplasmic space as well as the inclusion bodies formed inside the cell were extracted. Subsequently, these proteins were purified and detected by Western blot method. Results: Our results showed that the amount of reteplase extracted from periplasmic space was much lower than the extracted inclusion bodies and large quantities of the recombinant protein were present as inclusion bodies. Therefore, it was more efficient to use inclusion body extraction method for protein isolation and purification. Conclusion: We produced active reteplase after its expression in E. coli TOP10 and isolation of inclusion bodies produced the best results for purification and extraction of this protein.
Introduction :
The most important therapeutic goal in the management of acute myocardial infarction is early restoration of complete infarct artery perfusion after the occurrence of an acute coronary occlusion. Each year, between 1.5 and 2 million patients worldwide are admitted to hospitals with acute myocardial infarction 1. Today, the fibrinolytic agents are serine proteases which work by converting plasminogen to the natural fibrinolytic agent plasmin 2. Clinically, t-PA is a selective thrombolytic agent of choice for the treatment of acute myocardial infarction. It has the advantage of causing no side effects such as systemic hemorrhaging and fibrinogen depletion 3. t-PA contains 527 amino acid residues 4 and its correct folding requires the correct pairing of 17 disulfide bridges in the molecule 5,6. t-PA has five domains: F, EGF, P, Kringle 1 and Kringle 2 domains and the latter has 355 amino acids which is the active part of t-PA. Kringle 2 domain plus the first three amino acids of t-PA has been named reteplase which is available as a thrombolytic agent in the market 7.
The choice of an expression system for high level production of recombinant proteins depends on many factors such as cell growth characteristics, expression levels, intracellular and extracellular expression, post-translational modifications, biological activity of the protein of interest, as well as regulatory issues in the production of therapeutic proteins 8,9. The gram-negative bacterium E. coli is one of the most widely used hosts for the production of heterologous proteins and its genetic specifications are far better characterized than those of any other microorganism 10,11. The many advantages of E. coli have ensured that it remains a valuable organism for high-level production of recombinant proteins 9.
Reteplase has no carbohydrate side chains and thus can be produced in E. coli cells 7. This makes it a more valuable protein to express in E. coli as compared to t-PA. However, misfolding of this protein and other recombinant proteins expressed in E. coli has been a great concern. For example, expression of t-PA in pET15b has produced an inactive protein aggregated in the cell in the form of inclusion bodies. To overcome this problem, reteplase can be targeted to the periplasmic space which has less reducing environment as compared to cytoplasm 6. In this system, the vectors that carry arapBAD promoter can be used for the transformation of E. coli Top10 cells. Recombinant proteins are induced by adding L-Arabinose to the medium. In the present study, reteplase cDNA was inserted into pBAD/gIII plasmid and the presence of the expressed protein in periplasm and inside the E. coli Top10 cells was investigated.
Materials and Methods :
Plasmids and bacterial strains:Plasmids: recombinant pET15b/reteplase was previously prepared at the School of Pharmacy of Isfahan University of Medical Sciences 8. The pBAD/gIII plasmid and bacterial strains E. coli Top10 were obtained from Pasteur Institute, Iran.
Media and chemicals:Luria-Bertani (LB) media was prepared according to the guidelines in the laboratory manual offered by Sambrook and Russell 5. Screening based on antibiotic resistance was performed on LB agar plates containing ampicillin (100 µg ml-1) which was obtained from Sigma, Germany 12.
Transformation of E. coli DH5α cells with recombinant pET15b plasmids:One hundred l of CaCl2-competent E. coli DH5α (Cinnagen, Iran) cells were transformed with recombinant pET15b/reteplase plasmid. The transformed cells were spread on Luria-Bertani agar plates containing 100 mg of ampicillin (Sigma, Germany) per ml. After cultivation at 37°C for 24 hr, some colonies were selected for plasmid minipreparations using kit method (purchased from Fermentas Co., Poland). Plasmids were digested with Nco and BamH restriction enzymes to obtain reteplase cDNA inserts. On the other hand, pBAD vector was digested with Nco and Bgl enzymes. Vector and insert (molar ratio of 3:1, vector to insert) were ligated with T4 DNA ligase. Subsequently, E. coli Top10 bacteria were transformed using heat shock method (39C, 1 min) and were spread on LB agar plates containing 100 µg/ml ampicillin and then incubated overnight at 37°C. Afterwards, the obtained recombinant pBAD/gIII plasmids were sequenced (Biotechnology Kosar using the Analyzer Genetic Device and Capillary Base).
Expression of reteplase:A single clone of E. coli Top10 cells containing recombinant plasmid was cultured
in 5 ml Luria Bertani medium (1% tryptone, 0.5% yeast extract, and 1% NaCl) at 37°C, 180× g overnight. Then, 1 ml of this culture was inoculated into 100 ml of Luria-Bertani medium supplemented with ampicillin (100 µM) until OD600 of 0.4-0.6 was reached. Various concentrations of L-Arabinose was then added (0/2, 0/02, 0/002 and 0/0002%). Cells were incubated at 37°C, 180× g for an additional 4 hr and harvested by centrifugation at 5,000 g at 4°C for 10 min and the final product was stored at -20°C 13,14.
Protein extraction from the periplasmic space:Periplasmic proteins are proteins secreted into the periplasmic space located between the outer and inner membranes of E. coli. Proper secretion is possible only when the protein of interest has an N-terminal signal peptide that is cleaved following translocation. Cultured E. coli cells containing recombinant plasmid were centrifuged and the obtained pellet was resuspended in 30 mM Tris, 20% sucrose, pH=8.0, at 80 ml/gm wet weight. After incubation on ice for 10 min, 500 mM EDTA was added dropwise to the final concentration of 1 mM. The cells were placed on ice for another 5-10 min with gentle agitation. These cells were centrifuged at 8000 ×g for 20 min at 4°C and the supernatant was discarded. The pellet was resuspended in ice-cold 5 mM MgSO4, stirred for 10 min in ice bath and centrifuged at 8000 ×g for 20 min at 4°C. The supernatant (sample A) contained the periplasmic proteins and the remaining sediment (sample B) was stored at 4°C 5,15.
Preparation of inclusion bodies:For this purpose, about 25 gr of the cells were resuspended in 580 ml of 0.1 mol/L Tris and 20 mmol/L EDTA and homogenized with a shearing rod. Lysozyme at 0.25 mg/ml was added followed by incubation for 30 min on ice. Thereafter, centrifugation was carried out for 50 min at 4°C (15000 ×g). The pellets were resuspended in 300 ml of 0.1 mol/L Tris, 20 mmol/L EDTA and 2.5% V/V Triton X-100 and homogenized. After centrifugation, the pellets were resuspended in 300 ml. 0.1 mol/L Tris, 20 mol/L EDTA and 0/5% v/v Triton X-100 and homogenized. The samples were then centrifuged for 30 min at 4°C and 15000×g and the pellets were resuspended in 250 µL of 0.1 mol/L of Tris and 20 mmol/L EDTA 16,17. The prepared inclusion bodies were stored at -20°C.
Solubilization of inclusion bodies:Proteins were sequentially extracted from the prepared inclusion bodies by resuspension in 10 ml of Tris 25 mM, EDTA 10 mM, containing 1% Triton X-100 and guanidine HCl 6-8 M 18. The reducing agent and buffer components were separated by dialysis against 6 mol/L guanidine hydrochloride (pH=7) at 4°C 4,19.
Refolding and dialysis of reteplase:The solubilized samples were incubated in Tris 0.1 mol/L (pH=8.5); guanidine hydrochloride 6 mol/L; EDTA 2 mmol/L and DTT 0.3 mol/L for 3 hr at ambient temperature. We tested 3, 6, 12 and 24 hr retention times and our results showed that there was no difference between these times and therefore we used 3 hr for retention time. Subsequently, the pH of the solution was adjusted to 7 with concentrated hydrochloric acid. Refolding of the protein took place by dilution with 0.1 mol/L Tris (pH=10.5), 0.5 mol/L L-argenine, 1 mmol/L EDTA, 6-8 mol/L guanidine hydrochloride and 1 mg/ml bovine serum albumin. Then, the samples were incubated for 24 hr at 20°C. The reducing agent and buffer components were separated by dialysis against 6 mol/L guanidine hydrochloride (pH=7) at 4°C 4,19.
SDS-PAGE analysis:Harvested cells were washed with PBS buffer (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4, 0.05% Tween 20, pH=7.4). Next, the samples were boiled for 5 min at 70°C and electrophoreses on a 12% (vol/vol) SDS-PAGE analysis. Subsequently, the gels were stained with Coomassie brilliant G250.
Ion exchange chromatography of reteplase:In this project, the Q-sepharose Fast Flow was used for separation and purification of reteplase. Q-sepharose Fast Flow was equilibrated with Tris buffer (pH=9) and the protein samples were added to the column and eluted in a step wise manner with equilibration buffer containing 0.5 M to 1 M NaCl. This elution step was performed until no further proteins were eluted from the column. Then, the samples were analyzed using SDS-PAGE 12,20.
Western blot analysis:After SDS-PAGE, the proteins on the gel were transferred onto a nitrocellulose membrane. After incubating the blot with blocking buffer (5% non-fat dry milk in TBS) overnight at 4°C, tissue plasminogen activator antibody (anti t-PA, 1:250 to 1:1000 in blocking buffer) was added and incubated for 1 hr at room temperature. Subsequently, three washes with the blocking buffer were performed and anti-rabbit IgG-HRP conjugate (Roche, Germany) secondary antibody (diluted 1:5000) was added (incubated at room temperature for 1 hr). The membrane was washed three times with 20 ml TBS-Tween for 10 min. The TBS-Tween solution was then removed, the ECL solution was added (Amersham, USA) and the bands were detected on a common X-ray film.
Measurement of reteplase activity:Activation of plasminogen by reteplase was detected by Chromogenic Activity Assay Kit. Briefly, assay diluention (50 µL), plasminogen (10 µL), plasmin substrate (20 µL) and 20 µL of tPA standards or the samples were added to the supplied 96-well plate. The plate was incubated at 37C in a humid incubator and the absorbance at 405 nm for each sample was measured.
Results :
Cloning of reteplase:Recombinant pET15b plasmid containing reteplase sequence was digested with NcoІ and Bam HІ restriction enzymes while pBAD/gІІA plasmid was digested with NcoІ and BglІІ. Molecular weight of pBAD/gІІA is 4145 nucleotides and reteplase contains 1128 nucleotides (Figure 1). After ligation and transformation, the presence and correct orientation of the insert was evaluated using NcoІ and HindІІІ restriction enzymes. As shown in figure 1, the recombinant plasmids contained the correctly oriented insert. Sequencing of this plasmid also confirmed the presence of reteplase cDNA in the pBAD/ gІІA plasmid.
Expression of reteplase:After the transformation of E. coli Top10 bacteria with pBAD/gІІA plasmid containing reteplase gene, the effect of different concentrations of L-Arabinose (0.2, 0.02, 0.002 and 0.0002%) on reteplase expression was evaluated (1 hr, 2 hr, 3 hr and 4 hr at 25C, 30C and 37C incubation times and temperatures, respectively). The expressed proteins were electrophoresed using SDS-PAGE (Figure 2). Maximum production of reteplase occurred under the following condition: 0.0002% L-Arabinoze (as inducer), incubated at 37C for 2 hr.
Periplasmic and inclusion body extraction of the protein:Protein production was induced by L-Arabinose (0.0002% for 4 hr). Subsequently, proteins were isolated from the periplasmic space (samples A and B, supernatant and pellet, respectively) and were analyzed using SDS-PAGE. A protein with an estimated size of 39 kDa was observed in samples A and B (Figure 3).
Regarding isolation of inclusion bodies, after inducing with L-Arabinose 0.0002% for 4 hr, extraction of inclusion body was performed and the obtained pellets were analyzed by SDS-PAGE. As shown in figure 3, a strong 39 kDa band was observed.
Purification of reteplase:The obtained inclusion bodies were solubilized and refolded for 24 hr. After dialysis, purification of reteplase was performed using Q-sepharose fast flow column. This column was equilibrated with Tris buffer (pH=9) and proteins were eluted in a step wise manner with equilibration buffer containing 0.5 M to 1 M NaCl (speed of about 1 ml/min). A strong band was observed in fractions obtained after addition of 0.6 M NaCl (Figure 4).
Western blotting:After purification, the obtained reteplase was confirmed by Western blotting using anti-His antibodies (Figure 5).
Activity of reteplase:The activity of the obtained enzyme was analyzed using standard t-PA enzyme activity kit (Table 1). The change in the absorbance of pNA (para nitroaniline) in the reaction solution at 405 nm was directly proportional to the t-PA enzymatic activity. The concentration of tPA standard was 8000 units that is equal to 40 mg/ml. The concentration of samples was measured using standard curve of BSA (albumin serum) and it turned out to be 12 µg/µL.
Discussion :
Tissue plasminogen activator is an important enzyme for biotechnology industry due to its extended application in medicine 14. Therefore, the present research was conducted to produce a variant of this enzyme (reteplase) in E.coli Top10 bacteria using pBAD/gІІA plasmid. Induction of proteins using pBAD/gІІA system has been reported by many investigators 4,10,18,21 although none have studied the expression of reteplase in this system.
One important advantage of pBAD/gІІA plasmid used in the present study is the addition of signal sequence to the N-terminal segment of reteplase enabling it to be secreted into the periplasmic space. This would provide a better environment for proper folding of this enzyme 21. Several proteins such as pancreatic prokallikrein, human epidermal growth factor (hEGF) and protease inhibitors have been produced by secretion systems in E. coli periplasmic space 22-25. In this study, the amount of the expressed reteplase in periplasmic space was low. However, a high amount of this protein was present inside the cell as inclusion bodies. This might be due to high expression of proteins overwhelming the export machinery of cells for exporting inclusion bodies to the periplasmic space. Similar results have been reported by other investigators 8,14,24.
On the other hand, high levels of reteplase present as inclusion bodies prompted us to explore this system for protein production and purification. The main advantage of inclusion bodies is that they are mostly composed of recombinant proteins and can be easily isolated from the cell debris 8,22,25.
Data available on extraction and purification of reteplase are limited. Production of this protein in prokaryote cells is associated with the formation of insoluble mass and inactive form of inclusion bodies 7- 9,25,26. Thus, the main problem in purification process of inclusion bodies is optimizing the refolding and renaturation conditions by preventing the formation of inactive aggregates. Therefore, different refolding strategies have been used including the addition of arginine and oxidizing/reducing agents 27. Current methods for purification of the recombinant reteplase are Erythrina Trypsin Inhibitor (ETI) and affinity chromatography. These methods are considered as the expensive ones since they need more samples for purification. Our method of purification needed fewer samples and subsequent costs were less than other methods 18,28. There are several reports about the use of oxidizing/reducing gluthatione in refolding of recombinant proteins such as prochymosin, growth hormone and alkaline phosphatase 28.
Our result showed that the activity of our product was similar to the standard control. Qiu et al 22 and Lee and Im 29 have also produced tissue plasminogen activators and measured their activities. However, the unit activity of reteplase is defined differently as compared to other types of t-PA and therefore the results of these investigations cannot be compared with our study.
Conclusion :
In this study, to prevent the formation of inactive aggregates, oxidizing/reducing glutathione was used and after various steps of refolding and extraction from inclusion bodies, significant amounts of active protein were obtained. In conclusion, the active form of reteplase was obtained from the expressed protein in the form of inclusion bodies.
Acknowledgement :
This work was financially supported by the Research Council of Isfahan University of Medical Sciences (Grant no. 188048).
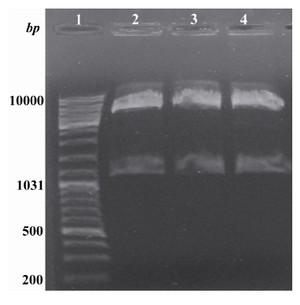
Figure 1. Digestion of the recombinant pBAD/gІІA plasmid with NcoІ and HindІІІ. Lane 1: Standard molecular weight marker. Lanes 2, 3 and 4: Obtained insert (1128 bp) and vector (4145 bp) after digestion with the above mentioned enzymes
|
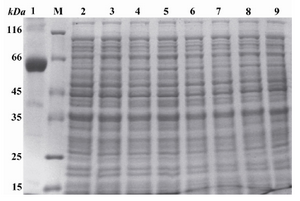
Figure 2. Induction of the expression of reteplase with L-Arabinose and separation of the obtained proteins using SDS-PAGE. Lane 1: Control sample, poly hydroxyalkanoat synthetase (PHC1) gene present pBAD/gІІІA vector induced with 0.0002% of L-Arabinose for 4 hr, producing a 60 KDa molecular weight band. M: Standard molecular weight marker. Lanes 3, 5, 7 and 9: pBAD/gІІІA plasmids without any insert induced with 0.2, 0.02, 0.002 and 0.0002 % of L-Arabinose and incubatd at 37C for 4 hr. Lanes 2, 4, 6 and 8: Recombinant pBAD/gІІІA plasmids containing reteplase sequence induced with 0.2, 0.02, 0.002 and 0.0002 % of L-Arabinose and incubated at 37C for 4 hr
|
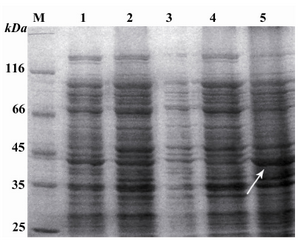
Figure 3. SDS-PAGE of the proteins extracted from periplasmic space and inclusion bodies. M: Standard molecular weight marker. Lane 1: Uninduced recombinant pBAD/gІІІA plasmids containing reteplase. Lane 2: Recombinant pBAD/gІІІA plasmids containing reteplase induced with 0.0002 mM L-Arabinose for 4 hr. Lane 3: Proteins present in fluid shock (sample A). Lane 4: Proteins present in the remaining pellet (sample B). Lane 5: Inclusion body sample
|
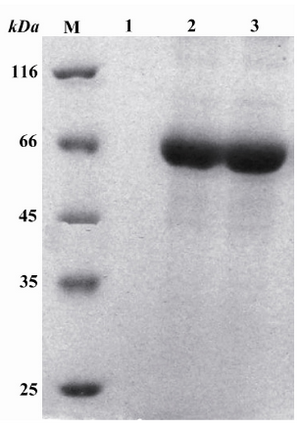
Figure 4. Purification of proteins extracted from inclusion bodies with Q-sepharose fast fast flow flow on SDS-PAGE gel. M: Standard molecular weight marker. Lane 1: The fraction obtained from 0 molar NaCl. Lane 2: The first fraction obtained from 0.6 molar NaCl. Lane 3: The second fraction obtained from 0.6 molar NaCl
|
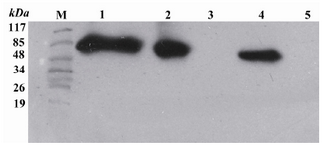
Figure 5. Western blot analysis by ECL method of extracts from TOP10 E. coli transformed with the recombinant pBAD/gІІІA plasmids containing reteplase using mouse anti His antibody. M: Pre-stained standard molecular weight marker. Lane 1: Refolded rpBAD. Lane 2: Purified rpBAD. Lane 3: TOP10 E. coli bacteria (negative control). Lane 4: t-PA standard (positive control). Lane 5: BSA 0.01% (negative control)
|
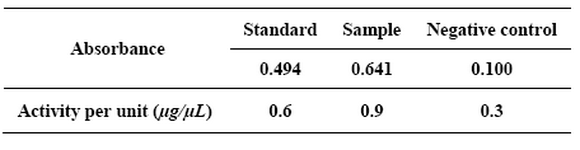
Table 1. The activity of the obtained enzyme with ELISA reader
|
|