Effect of Oral Supplementation of Biogenic Selenium Nanoparticles on White Blood Cell Profile of BALB/c Mice and Mice Exposed to X-ray Radiation
-
Yazdi, Mohammad Hossein
-
Department of Pharmaceutical Biotechnology, Faculty of Pharmacy and Biotechnology Research Center, Tehran University of Medical Sciences, Tehran, Iran
-
Varastehmoradi, Bardia
-
Department of Pharmaceutical Biotechnology, Faculty of Pharmacy and Biotechnology Research Center, Tehran University of Medical Sciences, Tehran, Iran
-
Mohammadi, Ehsan
-
Imam Khomeini Hospital, Tehran University of Medical Sciences, Tehran, Iran
-
Kheradmand, Erfan
-
Department of Pharmaceutical Biotechnology, Faculty of Pharmacy and Biotechnology Research Center, Tehran University of Medical Sciences, Tehran, Iran
-
Homayouni, Somayeh
-
Department of Pharmaceutical Biotechnology, Faculty of Pharmacy and Biotechnology Research Center, Tehran University of Medical Sciences, Tehran, Iran
-
 Shahverdi, Ahmad Reza
Department of Pharmaceutical Biotechnology, Faculty of Pharmacy and Biotechnology Research Center, Tehran University of Medical Sciences, Tel: +98 21 66482706; Email:Shahverd@sina.tums.ac.ir
Shahverdi, Ahmad Reza
Department of Pharmaceutical Biotechnology, Faculty of Pharmacy and Biotechnology Research Center, Tehran University of Medical Sciences, Tel: +98 21 66482706; Email:Shahverd@sina.tums.ac.ir
-
Department of Pharmaceutical Biotechnology, Faculty of Pharmacy and Biotechnology Research Center, Tehran University of Medical Sciences, Tehran, Iran
Abstract: Background: Radiation therapy is an effective method used for treatment of many types of cancers. However, this method can cause unwanted side effects such as bone marrow suppression. In this study, the effect of oral administration of biogenic selenium nanoparticles (SeNPs) on total and differentiated white cells profile of BALB/c mice exposed to X-ray radiation was investigated and compared with non-irradiated mice.
Methods: Sixty female BALB/c mice between six to eight weeks olds were divided into 4 test and control groups in two categories of normal and irradiated mice. In normal mice SeNPs administration was started from the day 0 and followed for a month. Irradiated mice were divided into three groups and were exposed to doses of 2, 4 and 8 Gy. After 72 hr of irradiation, the SeNPs treatment was started and continued for a month. Total and differentiated blood cells counts of both irradiated and non-irradiated groups were monitored during 30 days and the obtained results were compared. Also, the deposition of Se in different tissues and blood serum of normal mice was determined in normal mice after 30 days period of supplementation.
Results: In normal mice an increase in the count of neutrophils was observed after 30 days of supplementation. In irradiated mice, SeNPs supplementation led to increase in both lymphocytes and neutrophils counts especially in mice exposed to 2 and 4 Gys radiation.
Conclusion: Radiotherapy is categorized as an invasive method which can cause tissue damage and suppress the host immune defense. A restore of lymphocytes which was observed after SeNPs supplementation in irradiated mice can be highly interesting and provide cellular immunity against malignant diseases or other bacterial or fungal infections after radiotherapy.
Introduction :
Almost both radiotherapy and chemotherapy can cause considerable depression of the immune system, often by paralyzing the Bone Marrow (BM) and lead to a decrease of white blood cells, red blood cells, and platelets 1. The resulting anemia and thrombocytopenia are currently improved by blood transfusion. Synthetic G-CSF (granulocyte colony stimulating factor, e.g., filgrastim, lenograstim) is also being prescribed to improve the corresponding neutropenia (a decrease of the neutrophil granulocyte count below 0.5×109/L) in recent years 2. Cell depletion in patients undergoing chemotherapy or radiotherapy decreases the host immunity and leads to increased risk of infectious diseases caused by opportunistic microorganisms. These super-infections can significantly worsen the medical situation of cancer bearing patients 3. Despite the above side effects, chemotherapy and radiotherapy are still considered as important methods to delay the death of cancer bearing patients or increase their survival rates. However some natural products such as medicinal mushrooms like Trametes versicolor has been locally approved to counteract depression of the immune system in patients undergoing chemotherapy in Japan and they are even used as immunomodulatory agent in breast cancer immunotherapy 4.
Selenium (Se) as an important micronutrient ion has broad effects on biological systems, including antioxidant effects, cancer prevention, and antiviral activities 5. The biological effects of Se mostly depend on the incorporation of this metalloid into selenoproteins in the form of the amino acid selenocysteine 6. Deficiency in Se appears to result in immunosuppression, whereas supplementation with low doses of Se appears to result in augmentation or restoration of immunologic functions 7.
Elemental Se has been known to exist in various allotropic forms, as red amorphous form, black vitreous form, three (α, β, γ) of red crystalline monoclinic forms and grey/black crystalline hexagonal (also referred to as trigonal) form which is also the most stable form, and some more allotropes are discovered recently 8,9. Elemental Se is an insoluble metalloid compound which can be chemically or biologically produced at nano-scale 10. Since the toxicity reported for elemental Se (Se0) at nano size is lower than the toxicity of selenate (Se+2) or selenite (Se+4) ions, this nanoparticle may be a good candidate for replacement of other forms of Se in clinical practice 11,12. Recently, SeNPs have been prepared by biological methods using some bacteria such as Bacillus and lactobacillus bacteria 13,14. Also, the effects of the SeNPs on the iron homeostasis in sheep and their antifungal activity have been newly reported 15. However, based on our knowledge through literature review no report on the potential of SeNPs in recovery of white blood cells (WBC) depletion in mice exposed to radiation has been published yet. In the study presented here the effect of oral administration of SeNPs on the recovery rate of total and differentiated WBC of BALB/c mice exposed to harmful levels of X-ray radiation was investigated and compared with normal mice.
Methods and Materials :
SeNPs preparation:The preparation and purification of biogenic SeNPs were performed by a recently described method 14. For this purpose, the Lactobacillus plantarum (ATCC 8014) which was obtained from the Persian Type Culture Collection (Iranian Research Organization for Science and Technology, Tehran, Iran) was inoculated in 10 ml of DeMan-Rogosa-Sharpe (MRS) broth (Merck, Germany). The inoculated broth was incubated in a shaker incubator (200 rpm) for an overnight at 37°C. After an overnight selenium oxide (Merck, Germany) solution was added to the broth at the final concentration of 200 mg/L and incubation at 37°C was followed for 72 hr. Finally, the bacterial cells containing the SeNPs were removed from the culture medium by centrifugation at 4000×g for 10 min. The resulting pellets were washed with 0.9% NaCl solution by centrifugation (4000 ×g, 10 min) and were transferred to a mortar. By adding liquid nitrogen, the pellets were frozen which then were disrupted by a pestle. The resulting slurry was ultrasonicated at 100 W for 5 min and washed three times by sequential centrifugation (10000×g, 5 min) using 1.5 M Tris/HCl buffer (pH=8.3) containing 1% sodium dodecyl sulphate (SDS) and deionized water, respectively.
The pellets were suspended in deionized water, and the resulting suspension containing SeNPs and cell debris was collected. Approximately 4 ml of these suspensions was separately transferred to test tubes, and 2 ml of n-octyl alcohol was added to each tube. Then the assortments were shaken vigorously. The two mixed phases were totally separated by centrifugation at 2000×g for 5 min and were stored at 4°C for 24 hr. Following the time period, the generated SeNPs was observed at the bottom of the tubes and the cell debris remained between two phases. The lower and upper phases were discarded, and settled NPs were washed with chloroform, ethyl alcohol and distilled water, respectively 14. The obtained NPs were spherical in shape and their size was measured in the range of 20 to 150 nm. For further characterizations and biological experiments, the cleaned NPs were then re-suspended in deionized water and stored at 4C.
Animals:Sixty female inbred BALB/c mice between six to eight weeks of age and weighing between 25 to 30 g, were purchased from the Pasture Institute of Iran (Tehran, Iran). They were divided into 4 groups of tests and controls in two categories of X-ray irradiated and non-irradiated mice. Each experimental group contained 15 mice. The mice were kept in clean plastic cages, free access to water and food (standard mice pellet diet), kept on a 12:12 hr light and dark cycle during the experiment period. The temperature was controlled at 23±1oC and humidity at 55±10%. Even though the control mice were kept separated from the test groups, the temperature and humidity were the same as control mice and fed with the same food.
The effect of SeNPs supplementation on WBC profile of non-X-ray irradiated mice:In non-irradiated category of animals which contained 15 mice, SeNPs supplementation (100 µg/day) started from day 0 and followed everyday for a month. Furthermore, 15 mice were also considered as control group and were supplemented by phosphate buffer saline (PBS) solution under the same conditions.
Irradiation protocol and the effect of SeNPs supplementation on the WBC profile of X-ray irradiated mice:A linear accelerator instrument (Elekta Synergy system, UK) with photon energy of 6 MV was used to deliver sub-lethal irradiation doses of 2, 4 and 8 Gray (Gy) to the whole body. Thirty mice from the category of X-ray irradiated mice were divided into three groups each containing 10 mice and separately exposed to doses of 2, 4 and 8 Gy radio frequency radiation, respectively. Seventy two hr after irradiation each group was divided into two SeNPs or PBS supplemented subgroups each containing 5 mice. Then, SeNPs (100 µg/day) and PBS oral supplementation (1 ml) using standard gavage were started in mentioned SeNPs supplemented and non-supplemented animals (control group), respectively.
Blood sampling:The blood samplings have been carried out at different intervals (0, 7 and 30 days) after SeNPs administration in non-irradiated mice and at days of 10, 20 and 30 for those mice which exposed to the different doses of X-ray radiation. Sampling was conducted from the heart of mice under anesthesia induced by ether inhalation. One ml of blood from each mouse was collected in a proper blood collecting tube which contained anti-coagulating agent. Then the total and differentiated white cells profiles were analyzed with routine and conventional laboratory methods 16.
Analysis of Se in digested tissue and plasma:At the end of oral SeNPs supplementation in non-treated normal mice some animals from both test (received SeNPs) and control groups (received PBS solution) were scarified and different tissues were removed from their body. The tissues were incubated at room temperature in order to be dried and then 100 mg of each dried tissue was applied for digestion using a hazardous mixture of HNO3 and HCl (3:1) (10 ml) and kept for 48 hr under ventilation hood. Finally 1 ml of each sample supernatant was used for analysis of total Se by atomic absorption spectroscopy. The serum samples from the mice in both groups were also collected at the day 30th by routine method and further subjected to atomic absorption spectroscopy to determine Se concentrations.
Results :
Effect of SeNPs oral supplementation on the WBC profile of non-irradiated mice:As mentioned in methods and materials part, the blood samples were collected at different intervals (0, 7 and 30 days) from non-irradiated mice in both groups which received SeNPs or PBS solution (control group). No significant changes (Table 1) were observed in the levels of total WBC or other important white cells such as lymphocytes, monocytes in the mice which daily received SeNPs or PBS solution for a short period (1 week). In contrast after 30 days, a significant increase in total WBC was observed in SeNPs supplemented mice. Furthermore, the results of differentiated WBC analysis showed that the neutrophils counts were increased in normal mice which daily received SeNPs for 30 days (Table 1). Also, no increase in the lymphocytes counts was observed in SeNPs supplemented mice (100 µg/day) or mice which received PBS solution (control group) during this period (30 days).
SeNPs tissue distribution:According to the results of Se tissue distribution there was no considerable difference between the concentrations of Se in the collected sera or some removed organs of mice which received SeNPs or PBS solution for 30 days. Only a significant difference was observed for Se which was determined in the liver isolated from SeNPs supplemented mice compared to control group (Figure 1). This difference shows that some portions of SeNPs were deposited in the liver organ during oral supplementation of the SeNPs.
Effect of SeNPs oral supplementation on the WBC profile of irradiated mice:After irradiation and during the first weeks, total white blood cells decreased in all irradiated animals which received SeNPs or PBS solution (control group). In contrast in X-ray irradiated animals which were supplemented daily by SeNPs (100 µg/ml) for 30 days a considerable increase in total WBC counts was observed in comparison to control animals (Figure 2). In other word, 30 days Se supplementation led to a better recovery of WBC in BM suppressed animals especially in those test groups which received 2 and 4 Gy X-ray radiation. Differentiation analysis of WBC was also demonstrated that lymphocytes (Figure 3), neutrophils (Figure 4) and monocytes (Figure 5) counts were increased in blood circulation of irradiated mice which received SeNPs for 30 days in comparing to the control group even in test groups which exposed to a higher dose of X-ray (8 Gy).
Discussion :
Both radiotherapy operation and chemotherapy which are currently used to treat cancer disorders are myelosuppressive and cause serious side effects in cancerous patients. In the present study, the effect of oral administration of biogenic SeNPs on the recovery rate of WBC in BM suppressed mice exposed to X-ray radiation was investigated. Radiation causes apoptosis of BM stem cells and BM stromal cell damage, resulting in myelosuppression and characteristic pathologic and radiographic BM changes 17-19. Clinical studies have shown that the extent of radiation-induced BM injury depends on both radiation dose and volume of BM irradiated 20. Immune and hematopoietic systems as well as other organs such as gastrointestinal tracts, kidney, skin and lung are also sensitive to radiation and can be damaged during radiotherapy 21. The effects of ionizing radiation are mainly due to oxygen free radicals that are generated by their action on water. These highly reactive oxidants remove hydrogen atoms from fatty acids, lead to lipid peroxidation which causes changes in membrane permeability and fluidity and ultimately leads to cell death. The oxyradicals also induce DNA strand breaks and protein oxidation 22.
Se, as an essential micronutrient in animals, has three levels of biological activities: 1: trace levels are required for normal growth and development, 2: nutritional and supra nutritional levels can be stored, and homeostatic functions will be maintained, and 3: toxic levels can result in harmful effects 23. Heyland and his coworkers have suggested Se meal consumption as a protective antioxidative activity and a way of decreasing acute disease mortality 24. Se is involved in selenoprotein-type structures which include glutathione peroxidase (GPx) 25, P selenoprotein, and W selenoprotein. These proteins have a protective role against oxidant materials in body cells and cause an increase in the body's cell resistance such as immune cells against oxidative destruction 26,27 The most important action of Se is related to its antioxidant effects because it forms selenocysteine, which is the main part of the active center of the GPx 28,29.
In recent years, SeNPs have been of high interest to researchers due to their excellent biological properties which are similar to selenium ion even in lower dose with lower toxicity 12. In non-X-ray irradiated mice 30 days scheduled SeNPs oral supplementation (100 µg/day) only caused a considerable increase in neutrophils counts in test animals which received SeNPs. This finding confirms other previous reports published in literature on the effect of SeNPs on the homeostasis system 15. In contrast, when we prescribed the SeNPs for 30 days to X-ray irradiated mice for recovering of BM suppression it was observed that many types of important white cells, specially lymphocytes and neutrophils counts, can be significantly increased in SeNPs supplemented animals in comparison to the control group which received PBS solution only. A considerable change in lymphocytes' counts in non-irradiated animals and X-ray irradiated mice which all received SeNPs may be due to results of different cytokine profile in the mentioned animal groups.
In the present research, the effect of supplementation with biogenic and spherical SeNPs (ranging in size 20 to 150 nm) on the WBC recovery of X-ray irradiated and non-irradiated mice which all were supplemented by SeNPs (test groups) or PBS buffer (control groups) was investigated. The effects of additional factors such as the size variation, shape or dispersity of the SeNPs on the recovery of WBC counts in BM suppressed mice or normal animals have not been investigated in the current study and merit further investigation.
The researchers know that Se deficiency is an effective risk factor for the reduction of the lymphocyte reproductive potency, and demonstrated that the receptor of transferrin (which is effective in the reproduction of lymphocytes) will be reduced in the animals having Se deficiency 30-32. However, the reasons for this difference observed in differentiated WBC counts in non-X-ray irradiated animals and irradiated mice are currently unknown and merit further investigation. Lymphocytes are involved in cell mediated immunity and are highly required for the improvement of body defenses against tumors and opportunistic infections 33. SeNPs particles caused an increasing effect on the lymphocytes counts in mice which were exposed to harmful X-ray radiations, and this phenomenon maybe worthy of restoring the immune system of patients after radiotherapy and shift their immune defense to T helper type 1 cell (Th1) responses. There was not a significant difference between Se contents determined in many important organs and sera of non-X-ray irradiated mice which supplemented or non- supplemented by SeNPs for 30 days showing that no significant deposition (except to liver) occurred at dose of 100 µg/day in different vital organs such as brain, kidney and heart. The general toxicity of SeNPs has previously been studied by different groups and confirms that the toxicity of SeNPs is lower than Se ions 11,34 making the SeNPs suitable for further Se supplementation.
In the present work, the count of different kind of WBC in mice which irradiated with 2 Gy or 4 Gy doses of X-ray was considerably recovered after oral supplementation of SeNPs for 30 days. Higher X-ray irradiation (8 Gy) did not kill the animals but had serious side effects on BM and led to decrease WBC counts. This WBC depletion is not easily restored by daily administration of SeNPs for 30 days. It seems that longer administration periods for SeNPs or other routes of administration (i.e intra venous injection) may be useful for recovery of BM suppression in mice irradiated by X-ray at higher doses (8 Gy).
Conclusion :
Taken together our results indicate that a scheduled oral administration of SeNPs for 30 days can be used to recover BM suppression in X-ray irradiated mice and significantly increased the lymphocytes and neutrophils in BM suppressed mice. This increasing effect was observed only for neutrophils counts in blood sample collected from normal animals which were not exposed to any irradiation but supplemented for 30 days by SeNPs. It also can be concluded that regarding the increasing effects of SeNPs on all types of WBC especially lymphocytes in irradiated animal, SeNPs may be candidate for recovery of BM suppression in cancerous patients who receive invasive radiotherapy in the future.
Acknowledgement :
This work was supported by the Deputy of Research, Tehran University of Medical Sciences (Tehran, Iran). There is no conflict of interest in this work.
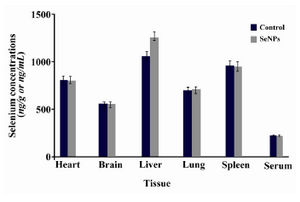
Figure 1. Distribution of Se in different tissues and sera of mice which received SeNPs for 30 days in comparison with PBS supplemented mice (control group). This figure shows that Se deposition was a little more only in liver tissue of SeNP administered mice compared to PBS control
|
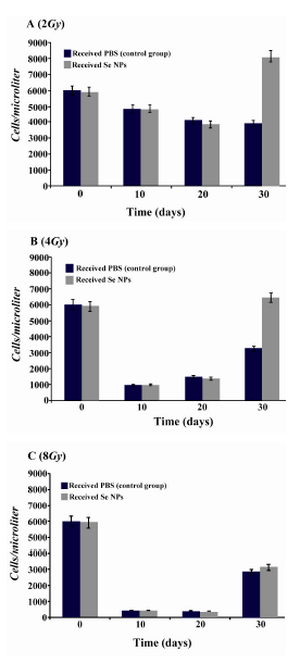
Figure 2. Total WBC counts in irradiated mice which received SeNPs or PBS buffer (control group) for 30 days. A: irradiated mice with 2Gy. B: irradiated mice with 4Gy. C: irradiated mice with 8Gy. Considerable increase was observed at the day 30th in total count of WBC in 2Gy and 4Gy irradiated mice
|
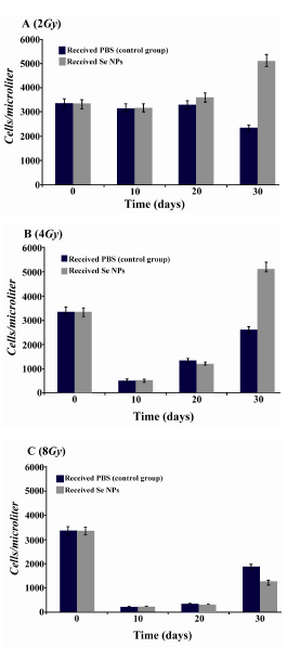
Figure 3. Lymphocytes counts in irradiated mice which received SeNPs or PBS buffer (control group) for 30 days. A: irradiated mice with 2Gy. B: irradiated mice with 4Gy. C: irradiated mice with 8Gy. Considerable increase was observed at the day 30th in the count of lymphocytes in 2Gy and 4Gy irradiated mice
|
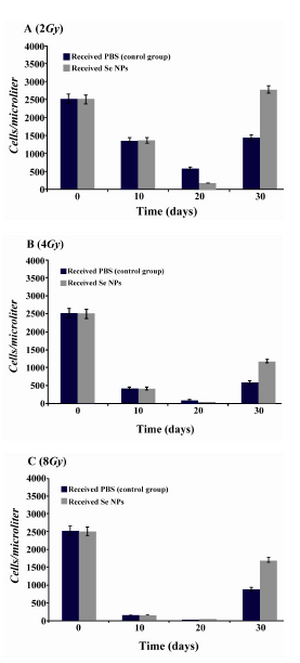
Figure 4. Neutrophil counts in irradiated mice which received SeNPs or PBS buffer (control group) for 30 days. A: irradiated mice with 2Gy. B: irradiated mice with 4Gy. C: irradiated mice with 8Gy. Considerable increase was observed at the day 30th in the count of neutrophil in 2Gy and 4Gy and even 8Gy irradiated mice
|
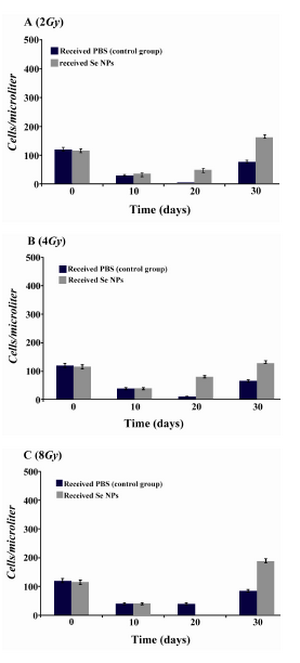
Figure 5. Monocytes counts in irradiated mice which received SeNPs or PBS buffer (control group) for 30 days. A: irradiated mice with 2Gy. B: irradiated mice with 4Gy. C: irradiated mice with 8Gy. Considerable increase was observed at the day 30th in total count of WBC in 2Gy and 4Gy and even 8Gy irradiated mice
|
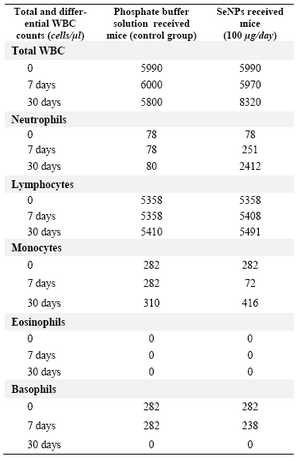
Table 1. Total and differential white cells counts in normal mice which supplemented or non-supplemented by SeNPs (100 �g/day) at different intervals. The considerable change in the count of total WBC and neutrophil have been observed in SeNP administered mice in comparison to non-supplemented mice in the 30th days of supplementation. WBC=White blood cell count
|
|