Cloning and Expression of Gumboro VP2 Antigen in Aspergillus niger
-
Azizi, Mohammad
-
Medical Biotechnology Department, Biotechnology Research Center, Pasteur Institute of Iran, Tehran, Iran
-
Industrial and Environmental Biotechnology Department, National Institute of Genetic Engineering and Biotechnology (NIGEB), Tehran, Iran
-
Yakhchali, Bagher
-
Industrial and Environmental Biotechnology Department, National Institute of Genetic Engineering and Biotechnology (NIGEB), Tehran, Iran
-
Ghamarian, Abdolreza
-
Medical Biotechnology Department, Biotechnology Research Center, Pasteur Institute of Iran, Tehran, Iran
-
Enayati, Somayeh
-
Medical Biotechnology Department, Biotechnology Research Center, Pasteur Institute of Iran, Tehran, Iran
-
Khodabandeh, Mahvash
-
Industrial and Environmental Biotechnology Department, National Institute of Genetic Engineering and Biotechnology (NIGEB), Tehran, Iran
-
 Khalaj, Vahid
Medical Biotechnology Department, Biotechnology Research Center, Pasteur Institute of Iran, Tehran, Iran , Tel: +98 21 66480780; Email: v_khalaj@yahoo.com
Khalaj, Vahid
Medical Biotechnology Department, Biotechnology Research Center, Pasteur Institute of Iran, Tehran, Iran , Tel: +98 21 66480780; Email: v_khalaj@yahoo.com
-
Medical Biotechnology Department, Biotechnology Research Center, Pasteur Institute of Iran, Tehran, Iran
Abstract: Background: Infectious Bursal Disease Virus (IBDV) causes a highly immunosuppressive disease in chickens and is a pathogen of major economic importance to the poultry industry worldwide. The VP2 protein is the major host-protective immunogen of IBDV and has been considered as a potential subunit vaccine against the disease. VP2 coding sequence was cloned in an inducible fungal vector and the protein was expressed in Aspergillus niger (A. niger).
Methods: Aiming at a high level of expression, a multicopy AMA1-pyrG-based episomal construct driven by a strong inducible promoter, glaA, was prepared and used in transformation of A. niger pyrG - protoplasts. SDS-PAGE and western blot analysis was carried out to confirm the expression of the protein.
Results: A number of pyrG+ positive transformants were isolated and the presence of expression cassette was confirmed. Western blot analysis of one of these recombinant strains using monospecific anti-VP2 antibodies demonstrated the successful expression of the protein. The recombinant protein was also detected by serum obtained from immunized chicken.
Conclusion: In the present study, we have generated a recombinant A. niger strain expressing VP2 protein intracellulary. This recombinant strain of A. niger may have potential applications in oral vaccination against IBDV in poultry industry.
Introduction :
Infectious Bursal Disease Virus (IBDV) belongs to the family of Birnaviridae, which consists of naked viruses characterized by a bi-segmented, double strand RNA genome and is the causative agent of a highly infectious disease affecting young chickens (Gumbro disease) 1. Current vaccination methods against IBDV are based on individual subcutaneous or intramuscular injection of inactivated or attenuated IBDV into birds. However, other administration routes such as oculonasal, oral or spraying are also common 2.
VP2 and VP3 are the major structural proteins of the viral capsid which form 51 and 40% of the total proteins, respectively 3. The VP2 protein with a molecular weight of ~42 kDa has been identified as the major host-protective immunogen of IBDV and contains main epitopes responsible for eliciting neutralizing antibodies 4,5.
Several studies have documented the efficient induction of protective immune response against IBDV following administration of recombinant VP2. For example, feeding broilers with VP2 expressing transgenic plants like rice seeds or Arabidopsis thaliana resulted in an antibody response against this protein 6,7. These examples along with few other studies support the idea that direct feeding of chicks with engineered hosts may be considered as a potential oral vaccination method 8,9.
Filamentous fungi, especially Aspergillus spp., are attractive production hosts for a wide variety of enzymes and metabolites. The ability to secret high amount of bioactive proteins, GRAS (Generally Regarded As Safe) status, rapid growth on inexpensive media, high level of biomass production and well-defined genetic manipulation techniques are main advantages for the industrial application of these organisms 10,11. For instance, the industrial strains of Aspergilli including Aspergillus niger, Aspergillus awamori and Aspergillus aculeatus have been successfully used in cost-effective production of various products in food and beverage, animal feed and paper-and-pulp industries 12,13. Furthermore, there are several reports on the application of Aspergillus biomass or culture extracts as prebiotic in poultry industry and various products like Fermacto® (http://www.pro-ag.com) are available 14.
Heterologous genes can be introduced to the fungal hosts via plasmids. Upon the integration of expression construct, various levels of the gene product can be expressed 15. Despite the lack of natural plasmids in filamentous fungi, an autonomous maintenance Aspergillus (AMA1)-based plasmid has been developed for the episomal expression of gene constructs in Aspergillus spp 16,17. The high frequency of transformation and relatively high copy number of plasmids in the nuclei facilitate a high level of expression 18.
Here, we have used an AMA1-based episomal construct to express VP2 protein of IBD virus in A. niger. The recombinant protein was reactive to commercial anti-VP2 monoclonol antibodies and serum from vaccinated chickens.
Materials and Methods :
Strains, plasmids and culture conditions: A. niger AB4.1, a pyrG derivative of A. niger N402 19, was used in expression experiments. E. coli Top 10 (Invitrogen) cells were used in DNA recombinant procedures. Plasmid pGEM-glaA comprising A. awamori glaA promoter and glaA termination signal was used for the preparation of intermediate expression construct. Plasmid pRG3-AMA1-NotI containing pyrG gene as a fungal selection marker was used for the preparation of final expression cassette.
Fungal strains were grown and kept on SAB agar or SAB agar medium supplemented with uridine and uracil (UU). Modified Vogel's medium 20 containing 1% w/v maltodextrin as the sole carbon source was used in expression analysis.
Construction of expression vector:The VP2 encoding sequence was cut from a previously prepared pPICZα-VP2 plasmid using HindIII and XbaI enzymes and cloned into HindIII/XbaI site of pGEM-glaA. To establish the correct reading frame, the resulting plasmid was digested with XhoI and then religated. This plasmid was called pglaA-VP2.2. To prepare the final construct, pAMA_VP2, a 3 kb NotI- fragment of pglaA-Vp2 containing the glaA promoter, VP2 coding sequence and glaA termination signal was cut and cloned into NotI site of pRG3-AMA1 (Figure 1).
Fungal transformation: A. niger AB4.1 cultures were grown for 20 hr in SAB-UU broth and protoplasts were prepared by gentle agitation of mycelia in a 5% (w/v) Glucanex (Novozymes, Switzerland) solution for 2 hr at 30oC. Transformation with pAMA-VP2 plasmid was mediated by poly ethylene glycol as described before 21. Positive transformants were selected on Vogel’s minimal medium lacking Uridin and Uralic.
Phenotypic analysis of the strains: For phenotypic analysis of VP2 transformant strain and the wild type strain (A. niger AB4.1 transformed with the empty pRG3-AMA1 plasmid), radial growth rates were determined by cultivation of 104 fresh spores from each strain on the center of SAB or modified Vogel’s agar plates at 30oC, 37oC and 42oC followed by serial measurement of colonies’ diameter for 5 days. Germination studies of wild type and VP2 transformant spores were carried out by incubation of fresh spores (104/ml) in SAB or liquid minimal medium up to 8 hr in 37oC. The number of germinated spores was counted in 2 hr intervals (triplicate experiments) and the percentage of germinated spores was calculated.
Expression analysis of vp2 in A. niger: A positive VP2 transformant was grown in 50 ml of inducing medium containing maltodextrin 1% (w/v) as the sole carbon source. The total fungal biomass was harvested after 24 and 48 hr of growth and then ground in liquid nitrogen. The resulting fine powder was re-suspended in a buffer containing 100 mM Tris-HCl pH=7.5 and 40 µl/ml protease inhibitor cocktail. The suspension was incubated on ice for 30 min and then centrifuged for 15 min at 4000 rpm. The resulting supernatant for each time point was collected and subjected to immunoblot analysis.
For immunoblot analysis, aliquots of the protein samples (30 µg) were loaded on 12% SDS-PAGE and the protein bands were electro-transferred to a nitrocellulose membrane. Immunodetection of VP2 in Western blot analysis was carried out using both mono-specific antiVP2 antibodies (IBDV92 and IBDV67, HyTest, Finland) and polyclonal serum obtained from immunized chickens. The VP2 signal on nitrocellulose membrane was detected by ECL plus kit (Amersham, USA) or routine DAB staining.
Results :
Construction of vp2 expression cassette:The expression construct was prepared in order to provide a high level of cytosolic expression of VP2 in A. niger. The VP2 gene segment with the size of 1355 bp was cut from an intermediate plasmid, pPICZα-vp2, and successfully cloned into HindIII/XbaI digest of pGEM-glaA. As it is shown in figure 1 (A and B), the final expression plasmid was prepared by insertion of the NotI fragment of pglaA-vp2.2 into NotI site of pRG3-AMA1 plasmid. Restriction analysis and DNA sequencing of pAMA-vp2 plasmid confirmed the correct cloning procedures (data not shown).
Transformation of A. niger AB4.1 strain: A. niger AB4.1 protoplasts were transformed with pAMA-vp2 plasmid. Several hundred pyrG+ transformants were obtained from a single reaction (3 µg plasmid). These transformants exhibited normal phenotype in minimal medium. A positive transformant was selected for further analysis.
To confirm that the expression cassette is present in this pyrG+ transformant, Plasmid DNA was extracted from this transformant and subjected to restriction mapping. The results confirmed that the isolated plasmid is intact and identical to original plasmid, pAMA-vp2 (data not shown).
The colonies radial extension rates (Kr, µm d-1) were determined as described earlier. The Kr value for VP2 transformant was 73±5 µm h-1 and for the wild type strain was 76±4 µm h-1 at 30°C in SAB agar medium. Comparison of radial growth means using the t-test did not show a significant difference between the growth rates of these two strains (p<0.05). Similarly, no significant difference was observed between the VP2 positive transformant and the wild type when grown on modified Vogel’s medium using different carbon sources including glucose and maltodextrin and different nitrogen sources like ammonium sulphate and peptone, or on complete medium at various temperatures (data not shown).
Germination studies of wild type and VP2 positive spores confirmed a similar pattern of germination type and the frequency of germinated spores. In SAB medium 65-70% of wild type or VP2 transformant spores were germinated after 4 hr incubation at 37oC, while the same percentage of germination in modified Vogel’s medium was achieved after 8 hr.
Expression analysis of VP2: Expression of recombinant VP2 was induced by cultivation of transformant spores in induction medium as described earlier. Following induction, 24 and 48 hr samples were taken and the lysates of both transformant and wild type strains were analyzed by SDS-PAGE. In Western blot analysis, a protein of ~42 kDa was reactive to anti-VP2 monoclonal antibodies and a positive serum obtained from immunized chickens (Figure 2A and B).
Densitometry analysis on SDS-PAGE gels demonstrated that the recombinant VP2 represents ~5% of total cell lyaste protein. This was equal to 0.23 mg/g of total biomass.
Discussion :
Current vaccination methods against IBDV using inactivated or attenuated virus are associated with some disadvantages including high costs, poor efficiency and emergence of new variants 7. Because of these limitations, attempts have been made to develop new subunit vaccines with fewer side effects. VP2 as the most important host protective immunogen of IBDV has been the focus of several studies. Despite the successful expression of VP2 in various eukaryotic systems, the purification of the recombinant protein is required 22. Wu et al have examined the capability of VP2 expressing transgenic rice seeds in oral immunization of SPF chickens against IBDV. Their results demonstrated that chickens can be immunized by direct feeding on the transgenic seeds, indicating the safe passage of antigen through the chicken GI tract 7.
Aspergillus species, particularly A. niger and A. oryzea are employed in many commercial processes as protein production hosts. Different expression systems have been developed to improve production yields of recombinant proteins in these industrial hosts 15. Moreover, as fermentation techniques are also well developed for these fungi, they can be grown in large quantities in inexpensive media 23.
Here, we have used an episomal plasmid to express VP2 antigen in A. niger. Self- replicating AMA1-based constructs are present at ca. 10 copies per nucleus and the level of expression of target gene is proportional to the copy number 18. The promoter region was selected from the highly expressed glucoamylase A (glaA) gene to direct a high level of VP2 production. This promoter has already been used for the successful expression of both homologous and heterologous proteins 24. Unlike the high levels of homologous protein production in Aspergillus, the heterologous protein production has been associated with low efficiency and generally the yields do not exceed few milligrams per liter without further optimizations 25. In the present study, the data obtained from a densitometry analysis showed that the initial levels of cytosolic recombinant VP2 is around 5% of total cell lysate proteins. Other investigators have reported similar yields in cytosolic expression of heterologous proteins, ranging from 1% to 10% of total soluble proteins 26,27.
Vp2 protein is hydrophobic and an appropriate host is needed for its efficient expression 28,29. Our results showed that A. niger is a suitable host for this purpose. The positive signal in western blot analysis of biomass crude extract using the immunized serum shows that the protein can be recognized by natural antibodies against IBDV indicating that the conformation of the recombinant protein is likely correct. To prove the antigenicity of the recombinant VP2 protein, direct feeding of SPF broilers with fungal biomass followed by serum analysis for detection of anti-VP2 antibodies is required. Our preliminary data with non-SPF chickens have shown that the feeding of chickens with the recombinant fungus prevents the maternal antibodies to decline (unpublished data).
As our final goal was to feed chickens by whole fungal biomass, no secretory signal sequence was added to the final construct, leading to the production of the target protein in cytosolic form. The oral administration of vaccines has some advantages over other routes. Oral vaccines are more convenient, cost effective, easy boosting, and can be applied in large scale vaccination programs 30,31. Comparing the transgenic plants, the cultivation of A. niger is more convenient and a large amount of biomass can be obtained in a short period. Cultivation of transgenic rice in the field or controlled green houses needs additional biosafety measures and large amount of spending, respectively. Furthermore, several studies have shown that Aspergillus meals (AM) can be used as a prebiotic in poultry industry. The benefits of AM include maturation of broiler GI tract, changing intestinal flora through the proliferation of useful bacteria, enhancement of resistance to pathogenic microorganisms and stimulation of growth by improving absorption of the feed ingredients 32,33.
Conclusion :
Here we have shown that filamentous fungus A. niger can be used as a suitable host for the production of VP2 protein of IBDV. This recombinant host is able to produce VP2 protein in cytosolic form. The recombinant protein was detected by antiVP2 specific antibodies and serum obtained from immunized chickens.
Acknowledgement :
Part of this research has been sponsored by the Pasteur institute of Iran through a grant awarded to VK.
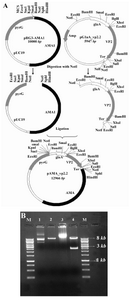
Figure 1. A) Schematic representation of expression cassette construction. Vp2 final fragment was cloned into NotI site of pRG3-AMA1; B) Restriction analysis of pAMA_vp2. M: Size marker. Lane 2: Undigested pRG3-AMA1-NotI (plasmid backbone). Lane 3: NotI linearized pRG3-AMA1-NotI (~ 10 kb). Lane 3: undigested pAMA_vp2. Lane 4: NotI digested pAMA_vp2. The backbone plasmid (~ 10 kb) and the glaA-vp2 fragment (~ 3kb) are present
|
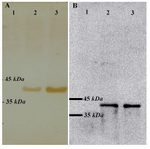
Figure 2. Western blot analysis on A. niger cell lysates using anti-VP2 antibodies. A) Detection of VP2 using polyclonal serum obtained from the immunized chickens. Lane 1: wild type strain; lane 2: vp2 transformant 24 hr sample; lane3: vp2 transformant 48 hr sample; B) Detection of VP2 using anti-vp2 monoclonal antibody. Lanes’ order is the same as A
|
|