Arylamine N-acetyltransferase 2 Polymorphisms and the Risk of Endometriosis
-
Fayez, Diman
-
Department of Biology, Faculty of Science, Science and Research Branch, Islamic Azad University, Tehran, Iran
-
Saliminejad, Kioomars
-
Department of Biology, Faculty of Science, Science and Research Branch, Islamic Azad University, Tehran, Iran
-
Irani, Shiva
-
Department of Biology, Faculty of Science, Science and Research Branch, Islamic Azad University, Tehran, Iran
-
Kamali, Koorosh
-
Reproductive Biotechnology Research Center, Avicenna Research Institute, ACECR, Tehran, Iran
-
Memariani, Toktam
-
Central Research Lab, North Khorasan University of Medical Sciences, Bojnurd, Iran
-
 Khorram Khorshid, Hamid Reza
Genetic Research Center, University of Social Welfare and Rehabilitation Sciences, Tehran, Iran, Avicenna Research Institute, Tehran, Iran, Tel: +98 21 22180138, 22432020 Fax: +98 21 22432021, E-mail: hrkk1@uswr.ac.ir, h.khorramkhorshid@avicenna. ac.ir
Khorram Khorshid, Hamid Reza
Genetic Research Center, University of Social Welfare and Rehabilitation Sciences, Tehran, Iran, Avicenna Research Institute, Tehran, Iran, Tel: +98 21 22180138, 22432020 Fax: +98 21 22432021, E-mail: hrkk1@uswr.ac.ir, h.khorramkhorshid@avicenna. ac.ir
-
Genetic Research Center, University of Social Welfare and Rehabilitation Sciences, Tehran, Iran
Abstract: Background: Human arylamine N-acetyltransferase 2 (NAT2) gene has a key role in xenobiotic metabolism through the conjugation of acetyl group to xenobiotic substances. NAT2 has been suggested as a susceptibility factor in endometriosis; however, the results of studies have been controversial. In this study, the association of NAT2 polymorphisms with susceptibility to endometriosis was evaluated in an Iranian population.
Methods: This is an association study and totally 141 women with diagnosis of endometriosis and 158 healthy women as control group were analyzed for NAT2 gene polymorphisms (C481T, A803G, G857A and G590A) by PCR-RFLP methods.
Results: The 590 GA genotype was significantly lower (p=0.001; OR=0.42, 95% CI: 0.25-0.71) in the patients (38.3%) than the control group (55.1%). The 590A allele was significantly lower (p=0.033; OR=0.69, 95% CI: 0.49-0.79) in the patients (31.2%) compared with the controls (39.6%). Analysis of haplotypes showed that NAT2 481C, 803A, 590A, 587A combination was significantly different between the case and control women (p= 0.029; OR=3.11, 95% CI: 1.13-8.52).
Conclusion: The NAT2 G590A SNP may be associated with susceptibility to endometriosis and the 590A allele may have a protective role in development of endometriosis. The NAT2 481C, 803A, 590A, 587A haplotype was associated with a higher risk of endometriosis in Iranian population.
Introduction :
Endometriosis, a benign gynecological and estrogen dependent disease, is the growth of endometrium outside the uterine cavity or myometrium, usually in the peritoneal cavity 1-3. The most important symptoms of endometriosis are back pain, infertility, dyspareunia, dysmenorrheal, dyschezia and pelvic pain 4. Several factors such as genetic, immune, endocrine and environmental factors have been associated with the pathogenesis of endometriosis 1,5-10.
The human arylamine N-acetyltransferase 2 (NAT2) gene, on chromosomal region 8p22 11, has a key role in conjugation of xenobiotic substances. NAT2 is involved in the initial biotransformation metabolism of aromatic amines and hydrazines, and catalyzes the transfer of an acetyl group from acetyl CoA to the nitrogen of the substrate 12. Some polymorphisms in NAT2 lead to amino acid substitution, which may result in impairment of the enzyme activity. Regarding NAT2, individuals are classified as rapid metabolizers if they have one or two wild-type alleles such as NAT2 *4 which is the most common allele. On the other hand, individuals are classified as slow metabolizers only if they carry two slow metabolizer variants such as NAT2 *5,*6 or *7 alleles. In a paper by Hein et al, they determined the NAT gene nomenclature 13. The alleles themselves are effectively haplotypes composed of several NAT2 polymorphisms, most typically assigned according to the status of the seven polymorphisms. NAT2 slow acetylation has been reported as a risk factor for bladder cancer 14,15, while fast acetylation has been implicated as a risk factor for colon cancer 16. Smelt and Mardon showed that there was a linkage disequilibrium between NAT1 and NAT2 genes 17.
The results of previous studies that evaluated the NAT2 polymorphisms with the risk of endometriosis are controversial. Therefore, in the current study, it was decided to investigate the association of NAT2 C481T (rs1799929), G590A (rs1799930), A803G (rs1208) and G857A (rs1799931) polymorphisms with endometriosis in an Iranian population.
Materials and Methods :
Subjects: In this case-control study, 141 Iranian patients with diagnosis of endometriosis were included. The diagnosis of endometriosis was accurately predicted preoperatively by clinicians. The severity of the disease (Stage I-IV) was scored according to the revised American Society for Reproductive Medicine (ASRM) classification. As the control group, 158 age-matched healthy women without endometriosis were selected. Absence of the endometriosis in the control group was confirmed by laparoscopic abdominal examination to inspect conditions other than endometriosis. Cases without confirmation of the disease by laparoscopy were excluded. Approval from Avicenna Research Institute's Ethics Committee was obtained. Written informed consent was received from the patients and controls. The study was done at Avicenna Research Institute, Tehran, Iran.
Genotype and phenotype determination: DNA was extracted from peripheral blood samples according to the salting out procedure. Genotyping of the four polymorphisms were performed using PCR-RFLP methods according to the previous study 16. Briefly, 546 bp fragment from exon 2 of NAT2 was amplified using the forward primer 5´-GCTGGTCTGG AAGCTCCTC-3´ and reverse primer 5´-TTGGGT GATACATACACAAGGG-3'. Following PCR amplification, digestion with the restriction enzymes KpnI, DdeI, BamHI and TaqI were performed to detect genotypes of the 481C/T, 803A/G, 857G/A and 590G/A polymorphisms, respectively. All enzymes were obtained from New England BioLabs and were used according to the manufacturer's instructions.
NAT2 acetylator phenotypes (slow, intermediate and fast) were predicted using the NAT2PRED Web server available at http://nat2pred.rit.albany.edu 18. NAT2P-RED is a web-server that predicts NAT2 acetylator phenotype from six polymorphisms of NAT2 C282T, T341C, C481T, A803G, G857A and G590A, without taking the extra step of reconstructing haplotypes for each individual 19. Because the genotypes for the NAT2 C282T and T341C were not determined, the wild type genotype for these polymorphisms was selected in the NAT2PRED web server.
Statistical analysis: Statistical analysis was performed with SPSS for Windows, version 13 (Chicago, IL, USA). Analyses of the allele frequencies and genotype distributions for the NAT2 polymorphisms in case and control groups were performed by χ² test and logistic regression analysis. All p-values were reported following two tailed statistical tests and values less than 0.05 were considered statistically significant. Each polymorphism was tested for Hardy-Weinberg equilibrium in the case and control population using χ² test. The SNPStat online software was used to estimate pairwise linkage disequilibrium (LD) and haplotype frequency (https://www. snpstats.net/snpstats).
Results :
Our results showed that of 141 endometriosis patients, 100 (71.0%) and 25 (17.7%) had stage I-II and III-IV of the disease, respectively; on the other hand, in 16 (11.3%) patients, the severity of the disease was not determined. The mean age of healthy and endometriosis group was 29±5 (range 19-44) and 31±6 (19-50) years, respectively that showed significant differences (p=0.03). The Body Mass Index (BMI) comparison between two groups shows significant differences (p=0.01). The differences of age and BMI between groups could affect the other results and therefore the genotype analysis was modified with regression logistic analysis based on age and BMI.
The distributions of genotype using χ² shown in both case and control groups for the NAT2 C481T, G590A, A803G and G857A polymorphisms were in Hardy-Weinberg equilibrium (p>0.05).
The genotype distributions and allele frequencies of the NAT2 C481T, G590A, A803G and G857A polymorphisms have been shown in table 1. Our results revealed that there was a significant difference in the genotype distributions and allele frequencies of NAT2 G590A polymorphism between the case and control groups. The NAT2 590 GA genotype was significantly lower (p=0.001; OR=0.42, 95% CI: 0.25-0.71) in the patients (38.3%) than the control group (55.1%). The NAT2 590A allele was significantly lower (p=0.033; OR=0.69, 95% CI: 0.49-0.79) in the patients (31.2%) compared with the controls (39.6%). This finding suggested that the NAT2 G590A polymorphism may be associated with susceptibility to endometriosis and the 590A allele may have a protective role in the development of endometriosis in Iranian women.
In contrast, no significant difference in the genotype and allele frequencies of the NAT2 C481T, A803G and G857A polymorphisms was found between the case and control groups.
The frequency of NAT2 acetylator phenotypes are shown in table 2. Slow phenotype was prevalent in the control group. Statistical analysis showed that the frequency of rapid acetylator phenotype was significantly higher in the endometriosis (34.8%) than the control (27.8%) group (p=0.025; OR=2.19, 95% CI: 1.10-4.36). Analysis of haplotypes showed that among different combinations of the four NAT2 SNPs (Table 3), only haplotype (481C, 803A, 590A, 587A) was significantly different between the case and control women (p=0.029; OR=3.11, 95% CI: 1.13-8.52).
Discussion :
C481T, G590A, A803G and G857A polymorphisms in exon 2 of NAT2 with the risk of endometriosis were investigated in an Iranian population. Our results revealed that there was significant difference in the genotype distributions and allele frequencies of NAT2 G590A polymorphism between the women with endometriosis and control groups. The NAT2 590 GA genotype and NAT2 590A allele were significantly lower in the patients than the controls (p=0.001 and p=0.033, respectively). This finding suggested that the polymorphism may be associated with susceptibility to endometriosis and NAT2 590A allele may have a protective role in the progression of endometriosis in Iranian women. On the other hand, the frequency of rapid acetylator phenotype was two-fold higher in the endometriosis than the normal controls.
The global Minor Allele Frequency (MAF) in the NCBI dbSNP database of the NAT2 C481T, G590A, A803G and G857A polymorphisms were T=0.27, A=0.27, G=0.32 and A=0.08, respectively. According to these frequencies, G857A is a relatively rare polymorphism.
The results of a few studies that evaluated the association of NAT2 polymorphisms and acetylator phenotypes with the endometriosis are controversial 19-23. There may be several explanations for the observed discrepancies. For example, in some studies only endometriosis patients with stage I-II or III-IV have been included. On the other hand, in some studies the acetylator phenotype for NAT2 enzyme was classified as slow and fast, while in the others, a three-category classification as slow, intermediate and fast has been used.
Baranova et al have reported that a higher proportion of women with stage I-II endometriosis were slow acetylators compared with controls, who were women undergoing termination of pregnancy with no evidence of endometriosis on physical or ultrasound examination (69 vs. 39%) 21. There was no statistically significant difference in acetylator status between women with stage III-IV endometriosis and the controls 20. Bischoff et al only investigated women with stage III-IV endometriosis and reported that 16/29 (55%) had the slow-acetylator phenotype 20.
Nakago et al investigated the relationship between endometriosis and NAT2 polymorphisms in a UK population 18. Homozygotes for the NAT2 *4 wild type allele are fast NAT2 acetylators, while heterozygotes with one wild-type allele and a variant NAT2 *5, *6 or *7 allele have reduced enzyme activity, and individuals with two variant alleles are slow acetylators. The NAT2 *4/*6 genotype was significantly more common among endometriosis patients (35.2%) than the controls (8.1%) or unaffected women (4.2%). Fast acetylators phenotype was significantly prevalent in endometriosis group (57.4%) than the controls (32.3%) or unaffected women (33.3%). Their results suggest that altered NAT2 enzyme activity may be a predisposition factor in endometriosis 18.
Babu et al investigated the NAT2 polymorphisms in 252 unrelated women with endometriosis and 264 controls of South Indian women 22. They found no differences between the frequencies of fast and slow acetylators in cases (34.9 and 65.1%) and controls (33.3 and 66.7%). Deguchi et al investigated the association between endometriosis and polymorphisms in the NAT1 and NAT2 in a Japanese population 23. Their results showed that the distribution of NAT1 and NAT2 allele and genotype frequencies were not significantly different between Japanese cases and controls.
Conclusion :
Investigation of NAT2 polymorphisms (C481T, A803G, G857A and G590A) showed that there may be an association between G590A polymorphism and risk for development of endometriosis in Iranian population, and the 590A allele may have a protective role in development of endometriosis. The NAT2 481C, 803A, 590A, 587A haplotype was associated with a higher risk of endometriosis.
Acknowledgement :
We would like to thank the patients, and other participants in this study. This work was supported by funds from the Avicenna Research Institute, Tehran, Iran (Project No: 890103-010). The authors declare that there is no conflict of interest.
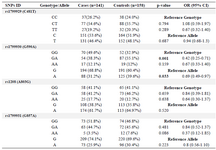
Table 1. Allele and genotype frequencies of NAT2 C481T, A803G, G857A and G590A polymorphisms in endometriosis patients and controls
Adjusted by age and BMI
|
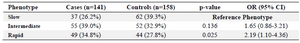
Table 2. The NAT2 acetylator phenotypes in cases and controls
|
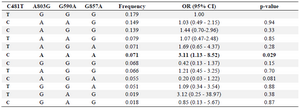
Table 3. Haplotypes frequency and association with endometriosis (n=299)
|
|