DNA Immunization as an Efficient Strategy for Vaccination
-
Bolhassani, Azam
-
Molecular Immunology and Vaccine Research Laboratory, Pasteur Institute of Iran , Tehran, Iran
-
 Rafati Yazdi, Sima
Ph.D., Molecular Immunology and Vaccine Research Lab, Pasteur Institute of Iran, Tehran, Iran, Tel: +98 21 66953311, Fax: +98 21 66465132, E-mail: s_rafati@yahoo.com ; sima-rafatisy@pasteur.ac.ir
Rafati Yazdi, Sima
Ph.D., Molecular Immunology and Vaccine Research Lab, Pasteur Institute of Iran, Tehran, Iran, Tel: +98 21 66953311, Fax: +98 21 66465132, E-mail: s_rafati@yahoo.com ; sima-rafatisy@pasteur.ac.ir
-
Molecular Immunology and Vaccine Research Laboratory, Pasteur Institute of Iran , Tehran, Iran
Abstract: The field of vaccinology provides excellent promises to control different infectious and non-infectious diseases. Genetic immunization as a new tool in this area by using naked DNA has been shown to induce humoral as well as cellular immune responses with high efficiency. This demonstrates the enormous potential of this strategy for vaccination purposes. DNA vaccines have been widely used to develop vaccines against various pathogens as well as cancer, autoimmune diseases and allergy. However, despite their successful application in many pre-clinical disease models, their potency in human clinical trials has been insufficient to provide protective immunity. Several strategies have been applied to increase the potency of DNA vaccine. Among these strategies, the linkage of antigens to Heat Shock Proteins (HSPs) and the utilization of different delivery systems have been demonstrated as efficient approaches for increasing the potency of DNA vaccines. The uptake of DNA plasmids by cells upon injection is inefficient. Two basic delivery approaches including physical delivery to achieve higher levels of antigen production and formulation with microparticles to target Antigen-Presenting Cells (APCs) are effective in animal models. Alternatively, different regimens called prime-boost vaccination are also effective. In this regimen, naked DNA is utilized to prime the immune system and either recombinant viral vector or purified recombinant protein with proper adjuvant is used for boosting. In this review, we discuss recent advances in upgrading the efficiency of DNA vaccination in animal models.
Introduction :
DNA vaccination is a relatively recent development in vaccine methodology. Al-though, DNA vaccine is a highly contro-versial issue, genetic material has been used for therapeutic purpose for the past fifty years. Scientists like Griffith had transferred DNA into cells of living animals in the early 1930. In 1943, Oswald Avery proved that DNA carries genetic information. After 1950, experiments were conducted using purified genetic material. Such experiments provided the evidence that direct injection of DNA results in the expression of the inoculated gene in the host even in the absence of vector. Regarding the DNA vaccine it was accident-tally discovered by scientists Tang and John-son (Express Healthcare). Among the many forms of nucleic acid vaccine that can be constructed, circular DNA plasmids are the simplest (1, 2). DNA vaccination involves im-munization with a circular DNA plasmid that contains the gene (or genes) that code for an antigen. Indeed, injection of free DNA (naked DNA) stimulates effective and long time immune responses to the protein (antigen) en-coded by the gene vaccine, which is being considered "the third generation vaccines". When plasmid DNA is injected into an individual, the plasmid is taken up by cells and its genetic information is translated into the immunizing protein. This enables the host immune system to respond to the antigen (3).
DNA vaccines have become an attractive approach for generating antigen-specific im-mune responses because of their stability and simplicity of delivery (4, 5). DNA vaccines can be easily prepared in large scale with high purity, repeatedly administered and are highly stable relative to proteins and other biological polymers (4). This strategy not only offers a relatively safe modality capable of inducing both cytotoxic T lymphocytes and antibodies, but also allows engineering of artificial im-munogens and co-expression of immuno-modulatory proteins. The resulting in vivo production of the protein after naked DNA injection, can involve biosynthetic processing and post-translational modifications (i.e., native protein form) (3). The efficiency of DNA vaccination against a pathogen can be affected by the choice of antigen and insertion of multiple antigens. In designing vaccine regimens, it is necessary to consider dose, adjuvants, time of injections and routes of vaccination (6). However, these vaccines are still experimental and have been applied to a number of bacterial, viral and parasitic models of disease as well as to numerous tumor models.
The active development of this technology only began after Stephen Johnston's group at the University of Texas, Southwestern Medic-al Center demonstrated that plasmid DNA can induce the formation of antibodies against an encoded protein in 1992. Johnston's group was able to show that when mice are inoculated with plasmid DNA encoding human growth hormone, the mice produce antibodies against the hormone. Then, another research group reported that a protective cell-mediated immune response against influenza virus was generated after immunization with plasmid DNA encoding an influenza virus protein. This study demonstrated that DNA-based immunization stimulates both compon-ents of the immune system and helped to establish that DNA immunization is capable of inducing a protective response against infection (DNA vaccine).
In spite of advantages of DNA vaccine strategies, a number of theoretical safety con-cerns may be considered for DNA vaccines. These include the fate of the plasmid in the vaccinated animals, the risk of the integration of vaccine DNA sequences into the genome of the host and the risk of inducing an anti-DNA immune response. These safety cases should be considered in vaccine design (7).
Two DNA vaccines were recently approved to be used in animals (horse and fish) pointing to the potential of this technology (8). The reasons for the failure of DNA vaccines to induce potent immune response
Acknowledgement :
Work in the authors’ laboratory is funded by grants from Pasteur Institute of Iran, Research Council of the Republic President-ship and UNDP/Work Bank/WHO/ TDR.
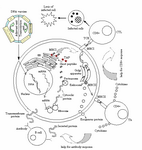
Figure 1. Molecular pathways of DNA vaccine by presenting the antigen to the T cells through the MHC class I and class II molecules. In endogenous pathway, the DNA plasmid enters the cell and nucleus, where the gene is transcribed into messenger RNA (mRNA). Then, mRNA is translated into protein by ribosomes in the rough endoplasmic reticulum (ER, not shown). In the cytosol the protein is cleaved by proteasomes, and the short peptides (contaning 8 to 10 amino acids) are transported into the ER with transport associated proteins (TAP1 and TAP2) and bind to MHC class I molecules. After binding, the complex is transported through the Golgi apparatus to the cell surface, where it can be recognized by cytotoxic T cells (CD8+) and stimulation of cell-mediated immunity occurs. In exogenous pathway, antigen-presenting cells take up extracellular proteins by either endocytosis or phagocytosis. MHC class II molecules in ER pass through the Golgi apparatus and enter acidified endosomes in which the foreign protein has been fragmented into peptides (Endolysosomal degradation pathway). The MHC�peptide complex is then brought to the cell surface, where it can be recognized by helper T cells (CD4+). Specific helper T cells recognize this antigen peptide/MHC class II molecule complex and are activated to produce help in the form of cytokines. These cytokines have many activities, depending on their types, helping B cell to produce antibody and helping cytolytic T lymphocyte (CTL) responses
|
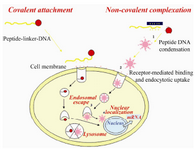
Figure 2. Peptide-based nucleic acid delivery systems must be able to: 1) tightly condense DNA into small, compact particles; 2) target the condensate to specific cell surface receptors; 3) induce endosomal escape and
4) target the DNA cargo to the nucleus for target gene expression
|
|