Development of an Immunoaffinity Method for Purification of Streptokinase
-
Karimi, Zohreh
-
Department of Biology, Faculty of Science, Alzahra University, Tehran, Iran
-
 Babashamsi, Mohammad
Avicenna Research Institute, ACECR, Tehran, Iran, Tel: +98 21 22432020; Email: babashams@avicenna.ac.ir
Babashamsi, Mohammad
Avicenna Research Institute, ACECR, Tehran, Iran, Tel: +98 21 22432020; Email: babashams@avicenna.ac.ir
-
Department of Immunochemistry, Monoclonal Antibody Research Center, Avicenna Research Institute, ACECR, Tehran, Iran
-
Asgarani, Ezat
-
Department of Biology, Faculty of Science, Alzahra University, Tehran, Iran
-
Salimi, Ali
-
Department of Immunochemistry, Monoclonal Antibody Research Center, Avicenna Research Institute, ACECR, Tehran, Iran
Abstract: Background: Streptokinase is a potent activator of plasminogen to plasmin, the enzyme that can solubilize the fibrin network in blood clots. Streptokinase is currently used in clinical medicine as a thrombolytic agent. It is naturally secreted by β-hemolytic streptococci.
Methods: To reach an efficient method of purification, an immunoaffinity chromatography method was developed that could purify the streptokinase in a single step with high yield. At the first stage, a CNBr-Ac-tivated sepharose 4B-Lysine column was made to purify the human blood plasminogen. The purified plasminogen was utilized to construct a column that could purify the streptokinase. The rabbit was immunized with the purified streptokinase and the anti-streptokinase (IgG) purified on another streptokinase substituted sepharose-4B column. The immunoaffinity column was developed by coupling the purified anti-Streptokinase (IgG) to sepharose 6MB–Protein A. The Escherichia coli (E.coli) BL21 (DE3) pLysS strain was transformed by the recombinant construct (cloned streptokinase gene in pGEX-4T-2 vector) and gene expression was induced by IPTG. The expressed protein was purified by immunoaffinity chromatography in a single step.
Results: The immunoaffinity column could purify the recombinant fusion GST-SK to homogeneity. The purity of streptokinase was confirmed by SDS-PAGE as a single band of about 71 kD and its biological activity determined in a specific streptokinase assay. The yield of the purification was about 94%.
Conclusion: This method of streptokinase purification is superior to the previous conventional methods.
Introduction :
A failure of homeostasis and consequent formation of blood clots in circulatory system can produce severe outcomes such as stroke and myocardial infarction. Pathological development of blood clots requires clinical intervention with fibrinolytic agent such as urokinase, tissue plasminogen activator and streptokinase (1).
Streptokinase (SK) is a non-protease plasminogen activator, produced by certain streptococci and certain bacteria which contain appropriate genetic material derived from streptococci of Lancefield groups A, C or G. Tillet (2) discovered that this bacterial protein caused the lysis of human blood clots. The fibrinolytic activity of streptokinase originates in its ability to activate blood plasminogen to plasmin, the enzyme that degrades fibrin cloth through its specific lysine binding site (3,4). Due to this property it is used in clinical medicine as a therapeutic agent in the treatment of thromboembolic blockage, including coronary thrombosis.
The mature streptokinase has a molecular weight of about 47 kD and was found to be composed of 415 amino acid residues (5). Secretion of streptokinase into the external medium is directed by a 26 amino acid signal peptide which is cleaved during the secretion process.
Production of natural and recombinant forms of SK and its purification by different chromatography methods, which are based on quantitative differences in solubility, electrical charge, molecular size and shape or non specific physical interactions with surfaces, has been studied by several workers (6,7). The growth of a β-hemolytic streptococcus was studied in continuous culture with pH as a limiting factor (8,9).
We have already reported the rate of SK production in a fed-batch culture and purification by affinity chromatography on acylated plasminogen with ρ-nitro phenyl guanidinobenzoate (NPGB) (10). The gene codes for streptokinase, from Streptococcus equisimilis (S.equisimilis) H46A was expressed in several heterologous gram positive and gram negative bacteria (11,12). A fusion recombinant streptokinase has been produced and purified it in a single step affinity chromatography using glutathione as the ligand (13).
In the present study, the issue of purification of recombinant Streptokinase (rSK) is addressed, for which a new immunoaffinity chromatography approach to produce active rSKC in a single step with high yield and stability was introduced.
Materials and Methods :
The materials used in the experiment includes; H46A (ATCC 12449, USA), E.coli DH5α and E.coli BL21 (DE3) pLysS (Invitrogen, USA), Todd Hewitt Broth (THB, HiMEDIA Laboratories), Trypticase Soy Agar (TSA, BBL, USA) Lysine monohydrochloride (Sigma Chemical, USA), pGEX-1.2-4T-2 (Avicenna Research Institute, Iran), Sodium Dodecyl Sulfate (SDS, Sigma Chemical, USA ) Hexyl resorcinol (Merck, Germany), NPGB (Sigma Chemical, USA), 3-amino-n-caproic acid (EACA, Sigma Chemical, USA ), Chromogenic substrate (S-2251, Chromogenix laboratories, Italy), Isopropyl-beta-D-thiogalactopyranoside (IPTG, Roche, Germany), Cyanogen bromide-activated Sepharose 4B (Sigma Chemical, USA), Sepharose 6MB –Protein A (GE healthcare, USA), Dimethyl pimelimidate-HCL (DMP, sigma-Aldrich, USA), Phenylmethylsulfonyl fluoride (PMSF, sigma Chemical, USA), serum albumin (sigma-Aldrich, USA), sheep anti-rabbit IgG (Avicenna Research Institute, Iran), o-Phenylenediamine (OPD, sigma Chemical, USA), Polyvinylidine difluride (PVDF, Roche, Germany), Enhanced Chemilumescent substrates (ECL, GE Health care, Biotech Buckinghamshir, UK), Chemicals for PAGE (Sigma Chemical, USA), Salts for buffers (Merck, Germany).
Preparation of streptokinase-sepharose column
S.equisimilis group C, strain H46A was grown in TSA and THB. The secreted SK purified by protected affinity chromatography (10). The purified streptokinase was then coupled to CNBr-activated Sepharose 4B. Briefly, 5 mg of purified SK was added per ml of the CNBr-activated Sepharose 4B resin in 0.1 M Sodium bicarbonate (NaHCO3, pH, 8.3) and after 2 hours at room temperature, the resin was washed with 0.2 M glycine, (pH=8.0) and then alternately for three times with 0.1 M sodium acetate, 0.5 M NaCL, (pH=4.0), and 0.1 M NaHCO3, (pH=8.3). The coupled streptokinase-sepharose was transferred to column, neutralized by Phosphate Buffered Saline (PBS) and stored in PBS-azide.
Production and purification of anti-streptokinase
Rabbit was immunized by subcutaneous injection of 1500 µl of an emulsion containing 25 µg of streptokinase and equal volume of complete Freunds adjuvant at 3 weeks intervals. Blood collected and serum was separated. Ten ml of sera was diluted 5 times with PBS, centrifuged at 4000 rpm for 10 min at 4oC to remove residual particles, and then was filtered. The supernatant was passed through a SK-Sepharose column at a rate of 12 ml per hour. It was washed by PBS and eluted with 0.2 M Glycine- HCl pH=2.5. Then it was neutralized by PBS. The purity was confirmed by SDS-PAGE.
Expression tests of fusion GST-SK in E.coli
SDS-PAGE analysis: E.coli BL21 (DE3) pLysS was transformed by pGEX-1.2-4T-2 ((pGEX vector with Glutathione S-Transferase (GST) tag and SK gene insert)) (13). Bacteria was grown in 5 mL LB broth to an OD of 0.6 (A600 nm) with vigorous agitation at 37°C. Fusion protein expression was induced by 0.1 mM IPTG and incubated for an additional 7 hr. One ml of the culture was collected at two hours intervals (1, 3, 5 and 7 hr), spun down and pellet was resuspended in 100 μl of 1x SDS sample buffer containing (7.5% Tris.base, 2 ml of 10% SDS, 1 ml Glycerol, 2 mg Bromophenol blue, 25 μl 2Mercaptoethanol). The cells were lysed using a strong vortex, heated at 100°C for 5 min, spind down and its supernatant was applied to 10% SDS- polyacrylamide gel electrophoresis. Proteins were stained with Coomassie blue and the band was visualized by destaining. In order to analyse the fusion GST-SK protein Molecular Weight (MW), the Bio-Rad MW marker, Cat. No. 161-0309 was used.
Western blotting: Sonicates of pre and post inductions of cloned GST-SK in E.coli and also a purified SK were run on SDS-PAGE. The gel was blotted on PVDF membrane using transfer buffer containing 25 mM Tris (pH=8.3), 192 mM glycine and 20% methanol at 100 v for 75 min at room temperature. Blotted membrane was blocked with 2.5% skim milk in TBST buffer (0.5 M NaCl, 0.02 M Tris pH=8.5, 0.05% tween 20) overnight at 4C. After twice washing with PBS-Tween, membranes were incubated for 1.5 hr at room temperature with 1 µg per ml rabbit antistreptokinase in 1% skim milk. After reactions with primery antibody, the membrane was washed three times with PBS-Tween and incubated with peroxidase conjugated sheep anti-rabbit IgG (Avicenna Research Institute) at a 1:2000 dilution in 1% skim milk. The membrane was then washed three times with PBS-Tween. The reaction was developed by ECL for 1 min and scanned.
Preparation of antistreptokinase-sepharose column
Cross-linking of antistreptokinase to protein-A sepharose 6MB:Twenty mg of purified anti-SK antibody in 10 ml of PBS was mixed with 1 ml of protein A-Sepharose 6MB continuously at 4oC and washed twice with excess of PBS. The beads rinsed with 8 ml of 0.2 M borate-NaOH, pH=8.6 and centrifuged for 1 min at 500×g. The washing was repeated with borate buffer two more times. Anti-SK was cross-linked to protein-A Sepharose by addition of 15.5 mg of DMP in 2 ml of 0.2 M triethanolamin buffer, pH=8.3. to 1 ml of the coupled bead. After rotation for 30 min at room tempreture, the cross-linking and washing steps were repeated twice to improve cross-linking effeciency. The rest of reactive amino groups were quenched by addition of 50 mmol/l glycine-HCL (pH=3). The antibodies cross-linked to beads were stored at 4oC in PBS containig 0.02% sodium-Azide Tween-20 (PBST) until use.
Purification of streptokinase on antistreptokinase-sepharose column: Two ml of 50% slurry of antibody-cross linked to protein A-sepharose was equilibrated with 20 ml of binding buffer [10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES)-NaOH, pH=7, 0.1 M NaCl, 2 mM PMSF]. Ten ml of recombinant streptokinase sample [sonicate of a 6 hours post induction of transformed BL21 (DE3) pLysS] was prepared in binding buffer and added on the bead. The binding occurred for 1 hr to over-night by continuously mixing.The bead was packed in column and sequentially washed with 30 ml of TBS (20 mM Tris-HCl, pH=7.5. 150 mM NaCl) with a flow rate of 1 ml/min and then with 10 ml pre-elution buffer (0.1 X TBS). The bound proteins were eluted with 5 ml of 50 mM sodium phosphate, pH=12. Five 1 ml fractions collected into tubes preloaded with 1 ml of cold 1 M Tris-HCl, pH=6. As soon as each fraction volume reaches 2 ml, contents of each tube were mixed to neutralize the eluate and placed on ice. The column was regenerated immediately after use by washing with 40 ml of 1 M Tris-HCl.
The activity of purified SK was measured by plasmin hydrolysis of chromogenic peptidyl anilide substrate (S-2251), procedure (14) with slight modification by us (13).
Results :
The culture of BL21 (DE3) PlysS', transformed by PGEX-1.2-4T2 construct induced by IPTG indicates that, the 71 KD fusion GST-SK protein is expressed increasingly (Figure 1). The western blot analysis of pre and post induction of this bacterial culture approved that the expressed protein is GST-SK (Figure 2).
The SK-Sepharose column constructed and the anti-SK antibody produced in rabbit and purified on the SK-Sepharose column. The purified antibody loaded on SDS-PAGE and the purity was confirmed (Figure 3).
The SK produced by H46a was purified by immunoaffinity column and two distinct bands of SK isotypes (48 and 45 kD) observed on SDS-PAGE (Figure 4).
The immune affinity column could purify the recombinant Fusion GST-SK to homogeneity as a single band of about 71 kD on SDS-PAGE (Figure 5). The yield of the purification was about 94% (Table 1).
Discussion :
Purification of SK have already been studied by various methods including; column chromatography on DEAE-cellulose followed by column electrophoresis in sucrose density gradients (15), a combination of ion exchange (DEAE-Sephadex A-50) and gel permeation (Sephadex G-100) chromatography (16). These methods required repeated chromatography to remove the impurities. As a result, the SK recovery yields found to be 40-60%. In other attempts SK was fractionated by ammonium sulfate and further purified by gradient elution from a DEAE-cellulose chromatography column (17). Several affinity chromatography methods were applied by different workers, including; insolubilized di-isopropyl fluorophosphates (DIP) plasmin as the affinity ligand (18). This method caused a 30% decrease in the streptokinase activity.
As mentioned earlier, SK was purified by a protected affinity chromatography method in which the ligand (plasmin) was acylated to protect its activation by SK (10). Even though the yield of purification by this method was found to be 95%, but acylation is not permanent and remains stable just for less than 4 hours. We have produced a recombinant SK tagged by a poly Histidin tail and purified both of the 6xHis-SK and GST-SK by affinity chromatography on NiNTA-agarose and glutathione-sepharose columns respectively, using thrombin for cleavage of the tags (unpublished). Thrombin is a costly enzyme, so this method is not cost effective for large scale purification. Recently, we have extracted and purified the native SK from H46a fermentation broth by chemical reduction method based on the specific structure of SK, lacking disulfide bridge (19). This method may not be applicable for recombinant SK purification from other bacterial sources, as they may secret proteins lacking disulfide bridges.
Conclusion :
Most of the mentioned methods have limitations, either thay are multisteps and often results in unacceptable losses of SK or inadequate removal of impurities. The present immunoaffinity method based on reversible linkage among antigen and antibody could purify the natural streptococcal SK and recombinant GST-SK in a single step with a yield of 94%. This method of SK purification is superior to the previous conventional methods as it does not require the addition of a tag to the recombinant SK for affinity purifications. Moreover, this method does not require any chemical modification.
In usual immunoaffinity method for purification of an antigen, the column consists of the immobilized antibody to the resin through its Fab region. So, the number of free Fab domains for antigen-antibody interaction is limited.
The present technique utilized protein A- sepharose 6MB as solid support to increase the chance of SK interaction with the Fab region of anti-SK, so antibody adhered to protein A from Fc region. A specific linker protected the anti-SK cleavage by elution buffer.
Acknowledgement :
We would like to thank research deputy of the Academic Center for Education, Culture and Research (ACECR) for financial support (Grant No: 1072-21).
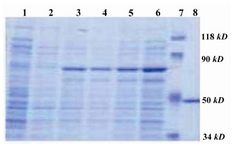
Figure 1. 10% SDS-PAGE; fusion GST-SK expression in E.coli, BL21 PLYsS: Lane 1: Pre-induction. Lane 3: 2 hr, Lane 4: 4 hr, Lane 5: 6 hr and Lane 6: 8 hr post-induction. Lane7: Protein marker (Fermentas SM0441). Lane 8: Pure SK
|
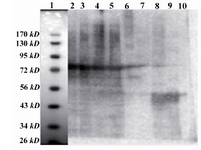
Figure 2. Western Blot of fusion GST-SK expression in E.coli, BL21 PLYsS: Lane 1: Protein MW marker (Fer-mentas SM0671). Lanes 2, 3, 4, 5 and 6: Cloned GST-SK in E.coli, after induction. Lane 7: BSA. Lanes 8, 9 and 10: Pure SK
|
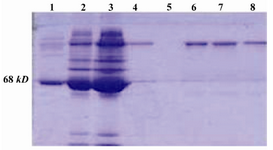
Figure 3. 10% SDS-PAGE; purification of anti-SK from rabbit sera, Lane 1: BSA, Lane 2: Flow-through, Lane 3: serum, Lanes 4, 6, and 7: Pure anti-SK, Lane 8: IgG
|
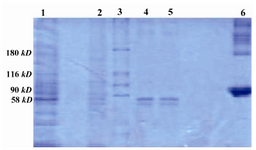
Figure 4. 10% SDS-PAGE; purification of SK from H46a, by immunoaffinity column Lane 1: Cultured H46a, Lane 2: Flow-through, Lane 3: Protein marker, Lane 4 and 5: Purified SK, Lane 6: BSA
|
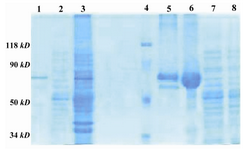
Figure 5. 10% SDS-PAGE; purification of recombinant fu-sion GST-SK from E.coli, by immunoaffinity column. Lane 1: Purified GST-SK 73 kD, Lane 2: Flow-through, Lane 3: Sonicate of E.coli, Lane 4: Protein marker (Fermentas SM0441), Lane 5: Hand made marker (Reduced IgG and BSA), Lane 6: BSA, Lanes 7 and 8: Non-transformed E.coli
|
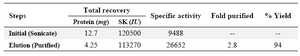
Table 1. Purification of fusion GST-SK
|
|