Ligation Independent Cloning of Polycistronic, Genetically Modified, HuMAb4D5-8 F (ab') 2, in Bacterial Plasmid
-
Farahmand, Leila
-
Department of Pharmaceutical Biotechnology, Faculty of Pharmacy, Biotechnology Research Center, Tehran University of Medical Sciences, Tehran, Iran
-
Iranian Center for Breast Cancer (ICBC), Academic Center for Education, Culture and Research (ACECR), Tehran, Iran
-
 Majidzadeh, Keivan
Keivan Majidzadeh-A, M.D., Ph.D., Iranian Center for Breast Cancer (ICBC), Academic Center for Education, Culture and Research (ACECR), Tehran, Iran, Zargham Sepehrizadeh, Ph.D., Department of Pharmaceutical Biotechnology, Faculty of Pharmacy, Biotechnology Research Center, Tehran University of Medical Sciences, Tehran, Iran, Tel: +98 21 66488980, 66959090 Fax: +98 21 66488980, 88027672 E-mail: kmajidzadeh@razi.tums.ac.ir; zsepehri@tums.ac.ir
Majidzadeh, Keivan
Keivan Majidzadeh-A, M.D., Ph.D., Iranian Center for Breast Cancer (ICBC), Academic Center for Education, Culture and Research (ACECR), Tehran, Iran, Zargham Sepehrizadeh, Ph.D., Department of Pharmaceutical Biotechnology, Faculty of Pharmacy, Biotechnology Research Center, Tehran University of Medical Sciences, Tehran, Iran, Tel: +98 21 66488980, 66959090 Fax: +98 21 66488980, 88027672 E-mail: kmajidzadeh@razi.tums.ac.ir; zsepehri@tums.ac.ir
-
Iranian Center for Breast Cancer (ICBC), Academic Center for Education, Culture and Research (ACECR), Tehran, Iran
-
AJA University of Medical Science, Tehran, Iran
-
Sepehrizadeh, Zargham
-
Department of Pharmaceutical Biotechnology, Faculty of Pharmacy, Biotechnology Research Center, Tehran University of Medical Sciences, Tehran, Iran
-
Esmaeili, Rezvan
-
Iranian Center for Breast Cancer (ICBC), Academic Center for Education, Culture and Research (ACECR), Tehran, Iran
-
Tabatabaei Yazdi, Mojtaba
-
Department of Pharmaceutical Biotechnology, Faculty of Pharmacy, Biotechnology Research Center, Tehran University of Medical Sciences, Tehran, Iran
Abstract: In recent years, recombinant monoclonal antibodies and their derivatives have emerged as important targeted therapy agents. Monoclonal antibodies are ex-tremely difficult to produce. So, the cost of production is very high and many people cannot afford these drugs. In this regard, choosing inexpensive and easy ways to manipulate production systems such as bacterial hosts to reduce the cost of manufacturing these critical components are considered as vital step for developmental issues in recombinant expression systems. We, therefore, at-tempted to generate a polycistronic construct of anti HER-2 F(ab')2 fragment antibody for insertion in an expression bacterial plasmid. Also some modifica-tions were made in the hinge region to express antibody F(ab')2 fragment in its authentic form preventing from multiple varieties of disulfide bond formation. Finally, synthesized construct was cloned in pET-32 Ek/LIC vector without using restriction enzyme digestion or ligation reactions. The results of this study show-ed that modified F(ab')2 fragment was simply and successfully inserted in Escherichia coli (E.coli) using the Ligation Independent Cloning technology.
Introduction :
Nowadays, targeted therapies have a sig-nificant impact on the management of various types of cancer and have a reputation for being a safe method with a higher specified action and lower side effects than the previous therapeutic agents (1). In recent decades during biotech-pharma investigation a few milestones have become manifested one by one, but it might be possible to say that none of them are as shining as recombinant monoclonal anti-bodies as targeted therapy agents (2).
To date, more than 30 monoclonal anti-bodies, including full length and their deriva-tives, have been approved by the Food and Drug Administration (FDA) for various indica-tions, and almost one thousand clinical trials are currently taking place (3). Despite the benefits of these therapeutic agents, the cost of treatment is drastically high and many pa-tients could not afford their prescriptions (4).
Antibodies are glycoproteins, which should be produced with a convenient glycosylation pattern that could possibly be achieved in mammalian expression systems such as Chi-nese Hamster Ovary (CHO) cells. The major potential glycosylation sites of antibodies are located in their constant regions that operate immune cell mediated Fc effectors such as antibody dependent cellular cytotoxicity (ADCC) (5). Since therapeutic function of some antibodies is not ADCC dependent, so their effect is glycosylation independent (6). In these cases, Fc segment of antibodies can be simply removed.
Antibody fragments will be beneficial for many clinical applications and have potential advantages over full length MABs. They re-tain the binding properties of their parent anti-body while their penetration into the tumor is more (7) and their potential antigenicity is less (8). Accurate formation of disulphide bonds; which has been overwhelmingly considered as a major post-translational modification as-pect, should be strongly spotted (9).
In this study, we attempted to design a gen-etically modified polycistronic F(ab')2 frag-ment gene and clone it in a bacterial vector by a simple and very fast method. Ligation Inde-pendent Cloning (LIC) is an easy and efficient method for cloning. Unlike most cloning pro-cedures, this method does not require purifi-cation, restriction enzyme digestion and liga-tion steps (10).
The chosen gene is related to a humanized anti-EGFR-2 (Human Epidermal Growth Fac-tor Receptor 2) antibody. This molecule binds to the extracellular domain of EGFR-2 (also known as HER-2) and prevents it from dimer-ization with other HER family receptors, in-hibiting the proliferation of human tumor cells with high potency (11).
Materials and Methods :
Strains, plasmids, and culture media
PCR enzymes and additional materials re-quired for gene amplification and gel electro-phoresis were purchased from Fermentas Company (Vinius, Lithuania). E.coli strain NovaBlue GigaSingles™ as initial cloning host and pET-32 Ek-LIC vector were obtain-ed from EMD Bioscience Inc. (San Diego, Canada). LB agar and broth which were used for culturing the strains were provided from Sigma (MO, USA); restriction enzymes were from Fermentas Company (Vinius, Lithu-ania).
Construction of expression vector
Polycistronic operon design: A polycistronic expression system, in which heavy and light chain of F(ab')2 fragment genes was inserted in-tandom on one cassette under the control of one promoter was designed. Ribosomal bind-ing site (RBS) and His•Tag and S•Tag se-quences were added between light and heavy chain sequences, upstream of heavy sequence. LIC site; which is encoded by sense primer; is designed to enable enterokinase (EK) cleav-age of all vector-encoded sequences from the expressed protein and the target protein will not have any non-native amino acid at the N-terminus after EK cleavage.
Hinge region modification: The design of CH1 (heavy chain constant domain1) gene segment, which encodes part of the antibody hinge region containing cysteines, was mod-ified to convert two cysteines out of three to alanines.
PCR amplification of F(ab')2 fragment gene: Designed DNA encoding F(ab')2 region of HuMAb4D5-8 was synthesized in bio S&T Inc (Canada). Then, F(ab')2 fragment se-quence was amplified by PCR using primers designed for cloning of the product into the pET32Ek/LIC vector. The sequences of the primers are as follows: Sense primer: 5' GAC GAC GAC AAG ATG 3' and Antisense primer: 5' GA GGA GAA GCC CGG TAA3'. PCR pri-mers were designed using Gene Runner Soft-ware v3.01 (Hastings Software Inc. Las Ve-gas, U.S.A) and synthesized by Cinnagen Inc. (Tehran, Iran).
The PCR was carried out in 50 µl volume containing 5 µl of 10×reaction buffer, 5 µl dNTPs (0.2 mM), 2 µl of each primer (12.5 mM/µl), 1.25 U Pfu DNA polymerases and
2 µl template (50 ng/ml). The amplifications were carried out using the thermal profile starting 2 min denaturation at 94 C, followed by 30 cycles consisting of 94C (1 min), 63C (45 s), and 72 C (2 min) with an additional extension time at 72 C (5 min). After ampli-fication, 10 µl samples were subjected into electrophoresis to confirm the presence of amplified PCR product.
LIC of F(ab')2 fragment gene: Based on LIC standard protocol compatible overhangs were generated on the amplified sequence by treat-ing purified PCR product with T4 DNA poly-merase in the presence of dATP. Then treated insert annealed into pET-32 Ek/LIC vector. The constructed cassette, pET-32 Ek-LIC/Hu MAb4D5-8 F(ab')2, was transformed into Nova Blue GigaSingles™ competent cells using calcium chloride method (12) and transfor-mants were cultured on LB agar containing tet-racycline (12.5µg/ml) and ampicillin (50µg/ml).
Confirmation of cloning: The presence of polycistronic cassette in cultured colonies was confirmed by following methods. Colonies were screened for the presence of inserts by colony PCR using vector-specific primers. Re-striction analysis was done using NcoI and XbaI enzymes. In this process, digestion was predicted to create three fragments with 3.7kb, 1.4 kb and 700 bp sizes. The proper sequence arrangement was confirmed by bidirectional sequencing.
Results :
Construction of expression vector
Polycistronic operon: Figure 1 shows the re-sult of polycistronic construct to co-express the heavy and light chains antibody F(ab')2 fragment under the control of one promoter. In order to achieve successful expression, the gene encoding the antibody F(ab')2 fragment was placed in the context of appropriate se-quences that allow transcription and trans-lation of the protein.
Hinge region design: The possibility of mul-tiple varieties of disulfide bond formation makes it possible to have many hinge linkages and probably some undesired forms of anti-body fragment (13). To this end, some modifi-cations were done in hinge region, converting the coding sequence of multiple cysteines into CysAlaAla (Figure 1).
Amplification of HuMAb4D5-8 F(ab')2 construct: The construct was synthesized accord-ing to the amino acid sequence of 4D5-8 anti-body and incorporating E.coli codon bias (GenBank accession numbers AY513485 and AY513484). Figure 2 illustrates the results of overhangs construction by PCR method using sense and antisense vector primers. F(ab')2 re-gion of HuMAb4D5-8 amplified by specific primers successfully. This vector was de-veloped for the direct cloning of PCR pro-ducts without restriction enzyme digestion or ligation reactions (LIC) (11).
LIC of HuMAb4D5-8 F(ab')2 construct: The resulting expression cassette was cloned into the framework of the E.coli plasmid pET-32 Ek-LIC vector at the LIC site, successfully. Each expression cassette contains the follow-ing components: 1-A T7 promoter, located on pET-32 Ek-LIC which controls transcription of light and heavy chain genes; 2-Two Ribo-somal Binding Sites (RBS) preceded each chain. The first RBS is related to pET-32 Ek-LIC and the second one which is inserted be-tween the locations of two chains, translates the second chain; 3-Two termination codons which end translation process, situated in both chains.
Confirmation of cloning: As presented in figure 3 colony PCR shows that the positive recombinant contains inserted fragment.
Figure 4, illustrates of expression cassette of pET-32 Ek-LIC/HuMAb4D5-8 F(ab')2. The F(ab')2 fragment was cloned at the LIC site. The F(ab')2 fragment is shown in polycis-tronic construct. Also figure 5, shows the re-sult of restriction digestion of pET-32 Ek-LIC/HuMAb4D5-8 F(ab')2. The size of expres-sion construct, pET-32 Ek-LIC/HuMAb4D5-8 F(ab')2, was 5.9 kb. The restriction map of the construct, using NcoI and XbaI enzymes, showed three fragments with expected sizes; 3.7 kb, 1.4 kb and 700 bp; that confirms a suc-cessful cloning of HuMAb4D5-8 F(ab')2 gene into pET-32 Ek-LIC expression vector.
Final analysis of the cassette, using se-quencing, showed successful cloning and cor-rect orientation of construct, downstream of enterokinase (EK) cleavage site (data not shown).
Discussion :
Regarding the invaluable benefits of anti- bodies and their fragments as therapeutic and diagnostic agents, especially in cancer diag-nosis and treatment, many investigations have been done in order to replace mammalian hosts with other more economical and im-proved systems (14).
Recently antibody fragments are emerged as advantageous molecules rather than full length antibodies. Batra et al reported that small antibody fragments like scFv, Fab or F(ab')2 exhibit better pharmacokinetics for tissue penetration in comparison with full antibodies. Also they provide complete bind-ing specificity because the antigen-binding surface is unchanged (15). Allison et al reported F(ab')2 fragment have higher affinity to bind surface antigens and also a longer serum half-life as compared with the smaller fragments (16).
Based on the above mentioned studies we predicted that F(ab')2 fragment, has many ad-vantages on full length abs and also score better than smaller fragments, as agents for chemotherapeutic or diagnostic purposes. So we attempted to generate HuMAb4D5-8 F(ab')2 gene construct for cloning.
Antibody F(ab') 2 fragments can be gener-ated by removing the Fc region of the IgG antibodies with proteolytic cleavage (17) or using recombinant Ig derived domains pro-duced in intended hosts (18). One of the major problems with expression of some antibody fragments such as F(ab')2 fragment is the cor-rect formation of protein folding. Jaenicke et al showed that the combinations of different disulfide bonds can be formed in a protein. These formations are dependent on the num-ber of cysteines in the native protein.
Also, the number of combinations in-creasingly rises with the number of disulphide bonds and finally enhances the possibility of heterogenicity (19). As F(ab')2 fragments nor-mally have three cysteines in their hinge re-gion, there would be fifteen different ways in which these bonds can be formed. If the bonds are formed at random, only a fifteenth of the molecules will fold properly. In this project, F(ab')2 fragment designed with some modifications in its hinge region in order to avoid intra-hinge disulfide binding. This modification results in proper configuration of the protein without misfolded ones.
The expression of antibodies and their frag-ments in different hosts such as Aspergillus niger (20), Arabidopsis thaliana wild-type plants (21) and Pichia pastoris (22) have been re-ported in some papers with variable amounts and characterizations. Higher levels of prod-uctivity, shorter production cycles, lower costs of downstream processes, no viral inac-tivation steps and non complicated culture media are important features which made us select bacterial cells as hosts for producing antibodies.
Some strategies of antibody gene cloning have been reported previously. Flamez et al reported the insertion of light and heavy chains of F(ab')2 genes on separate plasmids (23). Carter et al reported polycistronic Fab fragment construct and prepared the antibody F(ab')2 fragment by chemical reassociation of bacterial expressed monovalent fragments (24). Simmons L.C et al expressed a F(ab')2 frag-ment by a separate cistronic system. In this system, each antibody gene expression operon is located on one plasmid under the control of separate promoters (25).
As mentioned previously, we attempted to generate polycistronic F(ab')2 fragment con-struct. Polycistronic expression cassette is simpler to construct and expression can be controlled better than the separate cistronic systems because genes in polycistronic ex-pression cassette are under the control of one promoter instead of multiple promoters in separate cistronic vectors. This system is cap-able of generating transcripts greater than
10 kb in length because the gene expression is based on T7 RNA polymerase transcription and T7 RNA polymerase is known as a pro-cessive enzyme (11).
As mentioned earlier, LIC cloning system has many advantages as compared to many other methods of cloning. Weeks et al (26) and Botella et al (27) used LIC system successfully for “the rapid cloning and production of an affinity-tagged Small Ubiquitin Related Modi-fier (SUMO)” and “high-throughput analysis of gene expression in Bacillus subtilis” res-pectively.
Conclusion :
In this study the cloning of HuMAb4D5-8 F(ab')2 construct into the pET32Ek/LIC vec-tor was processed by LIC method in only some hours and very easily. So this method can be used for cloning of antibody gene frag-ments as a method of choice, eliminating problematic and time-consuming restriction digestion and ligation steps.
Acknowledgement :
This work is financially supported by Iran-ian Center for Breast Cancer (ICBC), Aca-demic Center for Education, Culture and re-search (ACECR). We thank Dr Ahmad Reza Shahverdi for his sincere collaboration in this study. We also thank Mrs. Nasrin Abdoli from ICBC and also Mrs. Farzaneh Barkhor-dary and Mrs. Zahra Khodayari from Pasteur Institute of Iran for their valuable cooperation and comments.

Figure 1. Polycisronic construction for cloning and expression of HuMAb4D5-8 F(ab')2 fragment gene in pET32Ek/LIC vector. Sense and antisense primers were added to the 5' and 3' ends of target construct to generate vector-compatible overhangs. The sense primer encodes the last four amino acids of the enterokinase (EK) cleavage to express the target protein on native form. Ribosomal Binding Site (RBS) is for efficient initiation of translation. His•Tag and S•Tag were used for simple detection and purification of target protein. Stop codons are used to terminate transcription of light and heavy chains. TGCGCCGCG is modified hinge region to encode CAA amino acids instead of multiple cysteines
|
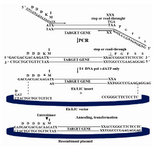
Figure 2. Diagram of the Ek-LIC strategy. After amplification with vector-specific primers that include the indicated 5' LIC extensions, the PCR insert is treated with recombinant T4 DNA Polymerase (+dATP), annealed to the vector, and the resultant nicked, circular Ek-LIC plasmid is transformed into competent E.coli. (User's Manual of Ek/LIC Kit)
|
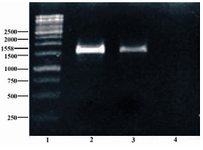
Figure 3. Colony PCR was done using specific primers of pET-32 Ek-LIC vector, followed by 1.5% agarose gel electrophoresis. Lane 1 shows 1 kb size marker (Fermentas, Vinius, Lithuania); Lane 2 shows synthesized sequence as positive control; Lane 3 shows colony PCR product; Lane 4 represents negative control
|

Figure 4. Expression vector for HuMAb4D5-8 F(ab')2.
A) Expression cassette of pET-32 Ek-LIC/HuMAb4D5-8 F(ab')2. The F(ab')2 fragment was cloned at the LIC site. B) The F(ab')2 fragment is shown in polycistronic construct
|

Figure 5. Restriction digestion of pET-32 Ek-LIC/ HuMAb4 D5-8 F(ab')2 construct by NcoI and XbaI enzymes which created three fragments;. Lane 1 shows the undigested cassette (5.9 kb); Lane 2 shows 1 kb size marker (Fermentas, Vinius, Lithuania); Lane 3 shows fragments created by NcoI & XbaI digestion (3.7 kb, 1.4 kb, 700 bp)
|
|