Plant-Based Recombinant Vaccines for Foot-and-Mouth Disease: A Meta-Synthesis
-
 Habibi-Pirkoohi, Maziar
Zist Pajoohan Baran Co, Afzalipour Incubator Center, Shahid Bahonar University of Kerman, Kerman, Iran, Tel: +98 9130416177; E-mail: maziar.habibi.p@gmail.com
Habibi-Pirkoohi, Maziar
Zist Pajoohan Baran Co, Afzalipour Incubator Center, Shahid Bahonar University of Kerman, Kerman, Iran, Tel: +98 9130416177; E-mail: maziar.habibi.p@gmail.com
-
Mohkami, Afsaneh
-
Research and Technology Institute of Plant Production, Afzalipour Research Institute, Shahid Bahonar University of Kerman, Kerman, Iran
Abstract: Background: Foot-and-Mouth Disease (FMD) remains a persistent global threat to livestock health and food security, particularly in endemic and resource-constrained regions. Conventional inactivated vaccines pose several challenges—including biosafety risks and dependence on cold-chain logistics. These limitations have prompted growing interest in plant-based recombinant vaccine platforms as innovative, scalable, and safer alternatives for FMD prevention.
Methods: This study employed a qualitative meta-synthesis approach, guided by the Barroso–Sandelowski method, to systematically extract, interpret, and integrate findings from 35 peer-reviewed empirical studies published between 2000 and 2025. The selected studies focused on the development and evaluation of plant-made vaccines targeting FMD. Thematic coding and interpretive synthesis were applied to identify recurrent patterns, challenges, and opportunities across the literature.
Results: The analysis yielded four dominant themes: (1) Platform Diversity: A variety of plant and algal expression hosts were used through transient or stable transformation systems, (2) Immunization Routes: Oral vaccination was noted for its logistical advantages and potential for mass immunization, though often requiring adjuvants to enhance immunogenicity, (3) Scale-Up Challenges: Key barriers included low recombinant protein yields, heterogeneity in post-translational modifications and high variability between production batches and (4) Regulatory Readiness: Despite encouraging preclinical data, most candidates have not progressed beyond experimental stages.
Conclusion: Plant-based recombinant vaccines represent a promising frontier in the fight against FMD, offering novel avenues for safer, more accessible immunization strategies. However, their transition from bench to field remains hindered by technical limitations in expression and purification, as well as institutional and regulatory gaps.
Introduction :
Foot-and-Mouth Disease (FMD) is a highly contagious viral disease affecting cloven-hoofed animals, including cattle, sheep, goats, and swine, and continues to cause substantial economic losses in the livestock sector worldwide 1. The disease’s rapid transmission, combined with its ability to severely impact animal productivity and trade, posing a major threat to global livestock health and trade. FMD is caused by Foot-and-Mouth Disease Virus (FMDV), a positive-sense, single-stranded RNA virus belonging to the family Picornaviridae and the genus Aphthovirus. The virus has seven distinct serotypes—O, A, C, Asia1, SAT1, SAT2, and SAT3—with no cross-protection between them, making vaccine design and regional immunization strategies particularly challenging 2,3.
Conventional vaccines are based on inactivated whole virus produced in BHK-21 cell lines under BSL-3 conditions, then chemically inactivated and formulated with adjuvants. These vaccines have played a major role in controlling FMD outbreaks, but suffer from critical limitations such as biosafety risks, cold chain dependency, limited duration of immunity, and the lack of Differentiating Infected from Vaccinated Animals (DIVA) capability 4,5. These shortcomings have fueled global interest in alternative platforms, such as plant-based recombinant expression systems, that can produce safe and immunogenic FMDV antigens without handling live virus 6,7.
While conventional inactivated vaccines have played a crucial role in disease control programs, their production involves several critical limitations. These include the requirement for high-biosecurity containment facilities to handle live FMD virus during manufacturing, as well as challenges in DIVA compliance, which complicates disease surveillance and outbreak management 8. Furthermore, conventional vaccines depend on cold-chain logistics for storage and distribution, which are particularly difficult to maintain in low-resource or remote settings, limiting vaccine accessibility and effectiveness. Production scalability also poses challenges, as the process is time-consuming and costly. Additionally, risks associated with incomplete viral inactivation present safety concerns that could hinder vaccine acceptance 10.
To address these challenges, plant-based recombinant vaccines have emerged as a promising alternative. In recent decades, recombinant subunit vaccines, especially those produced in plant-based expression systems, have gained increasing attention as a scalable and pathogen-free alternative 6,10. Plant-based platforms offer several distinct advantages: they are free from contamination by mammalian pathogens, allow for rapid scalability especially through transient expression technologies, and can produce Virus-Like Particles (VLPs) that closely mimic the native viral structure without containing any viral genetic material, thus enhancing safety 11. Several host plants have been explored for FMD antigen production, including Nicotiana benthamiana (N. benthamiana), a well-characterized model for transient expression, as well as alfalfa, maize, and lettuce, which hold promise for oral vaccine delivery due to their edible nature. These hosts have been successfully engineered to express key FMDV immunogens such as VP1, P1-2A, and P1-3C proteins, demonstrating promising immunogenicity and protection in various animal models 8,12.
To optimize vaccine efficacy, diverse immunization strategies have been investigated. Oral delivery systems utilizing transgenic or transiently expressed edible plant tissues provide an attractive needle-free, thermostable platform suitable for mass immunization campaigns. This approach reduces logistical constraints and improves animal welfare; however, oral vaccination faces challenges such as low mucosal immunogenicity and antigen degradation in the gastrointestinal tract 13,14. On the other hand, parenteral administration of purified recombinant proteins or VLPs extracted from plants has consistently elicited strong humoral and cellular immune responses, with several constructs demonstrating protective efficacy in experimental challenge studies 15,16. These findings suggest that prime-boost regimens combining oral priming with parenteral boosting could harness the advantages of both delivery routes 17.
Despite the clear promise and advances in plant-based vaccine platforms, significant translational challenges remain. These include the relatively low yields of recombinant proteins per biomass unit, the complexity of purification processes, and the need to ensure consistent post-translational modifications essential for antigen functionality 18. Additionally, regulatory uncertainty continues to hamper the development and commercialization of plant-made veterinary biologics, as harmonized guidelines and approval pathways are not yet well-established 19,20. Investment in downstream processing infrastructure and standardization of production protocols are necessary to overcome these hurdles and advance candidate vaccines toward commercial viability.
Given these opportunities and obstacles, this study undertakes a qualitative meta-synthesis to assess the state of the field; mapping technological progress, immunization strategies, and regulatory readiness to guide research and policy. This synthesis provides a structured framework to guide research, policymaking, and the advancement of next-generation FMD vaccines toward real-world application.
Materials and Methods :
Study design: This study employed a qualitative meta-synthesis approach to explore conceptual and thematic patterns in the development and evaluation of plant-based recombinant FMD vaccines, based on the framework developed by Sandelowski and Barroso, to integrate and interpret findings from empirical research on plant-based recombinant vaccines against FMD.
Search strategy and inclusion criteria: A comprehensive literature search was conducted across multiple databases—PubMed, Scopus, Web of Science, and Google Scholar—covering publications from January 2000 to May 2025. The following keywords and Boolean operators were used: ("plant-based" OR "plant-made" OR "transgenic plants") AND ("recombinant vaccine" OR "subunit vaccine" OR "virus-like particle") AND ("foot-and-mouth disease" OR "FMDV").
Studies were eligible for inclusion if they met the following criteria:
1. Published in peer-reviewed journals between 2000 and 2025
2. Reported empirical data on recombinant plant-produced antigens or VLPs targeting FMDV
3. Involved immunological evaluation in vitro or in vivo (mice, guinea pigs, pigs, cattle, etc.)
4. Provided sufficient methodological detail for qualitative coding
5. Written in English
Exclusion criteria included: conference abstracts without full text, review articles, non-English publications, and studies focused solely on non-plant-based expression systems.
Study selection and data extraction: Initial screening of titles and abstracts was followed by full-text reviews. A total of 35 studies were selected based on the inclusion criteria. For each study, the following data were extracted:
Year and authorship
Host plant species and expression system (transient vs. stable)
Antigen or epitope used (e.g., VP1, P1-3C, VLPs)
Immunization route (oral, subcutaneous, intranasal, etc.)
Animal model used and immunological outcomes
Yield, purification methods, and scalability remarks
Regulatory status or translational stage
Data analysis and thematic synthesis: Following Sandelowski & Barroso framework, data were analyzed using a four-stage process:
1. Extraction of conceptual findings: Conceptual units related to expression platforms, delivery route, scale-up, and regulatory readiness were identified.
2. Coding: Open and axial coding was performed using NVivo software to assign descriptive labels to recurring patterns and categories.
3. Development of thematic constructs: Coded segments were grouped into higher-order thematic categories reflecting the challenges and progress in the field.
4. Interpretive integration: Themes were synthesized across studies to generate conceptual insights, contradictions, and research gaps.
Out of 108 initial records, 26 met the inclusion criteria after full-text review. To enhance reliability, 20% of the dataset was double-coded by an independent reviewer.
Results :
The meta-synthesis of 26 studies yielded four major thematic domains central to the development and translation of plant-based recombinant vaccines against FMD. The full summary of studies, including platform types, expression hosts, antigenic targets, immunization strategies, and translational status, is presented in table 1.
The thematic constructs that emerged from interpretive synthesis are elaborated below (Table 2).
Platform diversity and expression systems: A consistent theme across the reviewed literature was the remarkable diversity of expression platforms employed for producing recombinant FMDV antigens within plant-based systems. These platforms encompassed both transient and stable expression approaches utilizing a broad spectrum of plant hosts, including N. benthamiana, alfalfa, maize, lettuce, and even the green microalga Chlamydomonas reinhardtii 9. Among these, transient expression via agroinfiltration in N. benthamiana was the predominant system used, largely due to its rapid production capabilities and relatively higher protein yields compared to stable transgenic plants. This method allows flexible, scalable production and rapid iteration of vaccine constructs, which is essential for timely responses to emerging viral strains 6.
On the other hand, stable expression systems, particularly in edible crops such as alfalfa, maize, and lettuce, have been primarily investigated for their potential in oral immunization strategies. These platforms offer significant advantages including the potential for needle-free administration, improved thermostability, and ease of delivery directly via feed, which are particularly valuable in resource-limited or field settings 21. However, these benefits come with trade-offs, notably lower antigen yields and the longer time frames needed for the development and selection of stable transgenic lines. Moreover, the bioavailability and immunogenicity of orally delivered antigens remain challenges, often requiring additional formulation or adjuvant strategies to enhance mucosal immune responses 10.
The types of antigens expressed in these plant-based platforms have predominantly included key structural proteins of FMDV such as VP1, P1-2A, and P1-3C. These proteins contain critical neutralizing epitopes and are fundamental for inducing protective immunity. Of particular interest has been the expression of full VLPs, which closely mimic the native virus structure without containing infectious genetic material. VLPs have consistently demonstrated superior immunogenicity compared to individual proteins, eliciting robust humoral and cellular immune responses. However, their production is inherently complex, often requiring the co-expression of multiple structural proteins through multi-vector systems or intricate subgenomic constructs to facilitate correct assembly and stability of the VLPs 11.
Immunization routes and immunogenicity profiles: Three primary delivery methods were identified across the reviewed literature: oral, Subcutaneous (SC), and intranasal administration. Among these, oral delivery using lyophilized or fresh plant material expressing FMDV antigens emerged as a frequently investigated strategy. Oral vaccination is particularly attractive due to its ease of administration, which does not require trained personnel, making it highly suitable for mass vaccination campaigns especially in low-resource or rural settings. Additionally, oral vaccines based on plant tissues offer enhanced thermostability, which significantly reduces reliance on cold-chain logistics—a major limitation of conventional vaccines 14. Despite these advantages, many studies have noted challenges associated with the oral route, primarily related to the relatively low immunogenicity at mucosal surfaces. This has driven research into the incorporation of mucosal adjuvants, such as Cholera Toxin B subunit (CTB) or heat-labile enterotoxin, which have shown potential in enhancing antigen uptake and stimulating stronger local immune responses 22.
In contrast, parenteral delivery—specifically subcutaneous administration—of purified recombinant proteins or VLPs derived from plant expression systems consistently produced robust systemic immune responses. Experimental models including mice, guinea pigs, and cattle demonstrated high titers of specific IgG antibodies and potent virus-neutralizing activity following parenteral immunization 23. This systemic immunity is crucial for effective protection against FMDV infection, which spreads rapidly through the bloodstream. The strong immunogenicity observed with plant-derived antigens administered subcutaneously supports their further development as practical vaccine candidates 24.
Additionally, a smaller subset of studies explored prime-boost immunization regimens that combined plant-derived antigens with existing commercial vaccines. These regimens aimed to synergistically elicit both mucosal and systemic immunity, potentially providing broader and longer-lasting protection 1. For instance, oral priming with plant-expressed antigens followed by parenteral boosting has been proposed to harness the advantages of mucosal tolerance induction along with systemic antibody production, addressing the limitations inherent in either delivery method alone. Although promising, such combination approaches require further optimization and evaluation in large-scale trials to fully establish their efficacy and safety profiles 13.
Scale-up barriers and downstream processing challenges: Despite promising lab-scale results, most studies identified significant barriers to scale-up. These included low recombinant protein accumulation (often <100 µg/g fresh weight), complex purification protocols, and inconsistent antigen stability 9,11. Stable expression systems were particularly limited by variable expression levels across generations and lines 10.
Moreover, glycosylation patterns in plant systems often differed from those in native mammalian hosts, raising concerns about epitope authenticity and immunogenicity. Only a few studies reported process optimization steps, such as codon optimization, chloroplast targeting, or fusion to stabilizing peptides (e.g., elastin-like polypeptides) 15.
Regulatory readiness and translational gaps: Although several plant-based FMD vaccine candidates have demonstrated significant immunological promise through preclinical studies, none have yet successfully navigated the complex pathway to regulatory approval or achieved commercial deployment. A major impediment to translation lies in regulatory challenges, which include the scarcity of manufacturing facilities compliant with Good Manufacturing Practice (GMP) standards specifically tailored for plant molecular farming. This gap hinders the ability to produce consistent, quality-assured vaccine batches at scale, which is a prerequisite for regulatory acceptance 25. Furthermore, the absence of harmonized regulatory frameworks and clear guidelines for plant-derived veterinary biologics complicates the approval process, as regulatory bodies currently lack standardized criteria for evaluating safety, efficacy, and environmental impact of these novel biologics 26.
Another significant regulatory hurdle involves the limited investment and execution of validation studies that demonstrate DIVA compatibility—a critical feature for disease surveillance and control programs. Without robust DIVA data, widespread adoption of plant-based vaccines remains unlikely, as authorities require clear differentiation capabilities to prevent false positives during outbreak investigations 2.
Only a handful of studies have explicitly addressed regulatory planning and navigated these challenges, revealing a critical gap between laboratory-based innovation and real-world vaccine deployment 10. This gap underscores the need for early integration of regulatory strategy during the vaccine development pipeline to streamline translation efforts. In addition to regulatory concerns, Intellectual Property (IP) constraints and complex biosafety approval processes for the cultivation and use of transgenic plants present latent but formidable barriers to conducting field trials. The stringent regulatory scrutiny on Genetically Modified Organisms (GMOs) often leads to lengthy approval timelines and adds financial and logistical burdens on developers. These factors collectively contribute to the slow progress in advancing plant-based FMD vaccines from experimental proof-of-concept to practical, field-ready solutions 27 (Table 2).
Immune response profiles: Limitations to meta-analysis: Substantial variation in immunogenicity outcomes across studies on plant-based FMD vaccines precluded a formal statistical meta-analysis. Several studies have demonstrated the ability of plant-derived antigens to elicit humoral immune responses in animal models. For example, Huang et al 28 reported robust IgG responses following immunization with epitope–HBc fusion proteins expressed in transgenic tobacco, with titers ranging from 1:800 to over 1:32,000 depending on dose and schedule. Similarly, Alsharifi et al and Veerapen et al documented promising results using VLPs expressed in N. benthamiana, yet they noted wide inter-study variability in both immunoglobulin titers and protection outcomes 6,11.
Protection rates post-challenge also varied considerably, ranging from 60 to over 90% 4,25, with differences attributed to antigen format (e.g., VP1, polyprotein, VLP), host species, and challenge protocols. Moreover, the diversity in assay types (ELISA, neutralization tests), sampling time-points, units of measurement (e.g., optical density vs. endpoint titers), and lack of consistent reporting of key statistical parameters (e.g., standard deviations, sample sizes) significantly limits data comparability.
As a result of this methodological heterogeneity, and the frequent omission of raw or normalized data, a quantitative meta-analysis could not be performed. Instead, findings were synthesized narratively to reflect general trends and recurring limitations in the current literature, aligning with recommendations from recent reviews on recombinant vaccine development 3,7. The absence of standardized reporting frameworks across preclinical studies remains a critical barrier to cross-study comparison and evidence-based evaluation of vaccine efficacy.
Patent landscape and IP barriers: Analysis of the IP environment surrounding plant-based recombinant vaccines for FMD reveals a fragmented and highly territorial patent landscape. A significant number of patents target VLP platforms, synthetic epitope designs, and plant-based expression systems such as N. benthamiana and transgenic maize 29. Several studies 30,31 indicate a growing trend in patent filings related to scalable production methods and multi-epitope constructs, especially those designed for improved immunogenicity and thermostability (Table 3).
Despite these innovations, patent ownership is largely concentrated within a limited set of institutions and jurisdictions, primarily in high-income countries. This concentration poses challenges for technology transfer and commercial deployment, particularly in low- and middle-income regions where FMD is endemic. Moreover, the lack of standardized or centralized patent databases across countries complicates comprehensive assessment of Freedom-To-Operate (FTO) for developers. Licensing requirements for core technologies—such as VLP scaffolds or proprietary promoters—may further impede the entry of smaller biotech companies into the field. Only a limited number of vaccine candidates described in the literature explicitly address IP accessibility or include strategies for open licensing or public-sector partnerships. These findings underscore the potential influence of patent dynamics on vaccine availability and affordability across different regions.
Clinical development stage of candidate vaccines: A comprehensive review of published studies indicates that most plant-based recombinant vaccines for FMD have not progressed beyond the preclinical stage. Experimental evaluations to date have primarily involved murine models, guinea pigs, and target livestock species such as cattle, sheep, and pigs 11,22,30. These studies have consistently demonstrated the ability of plant-derived VLPs, VP1 constructs, and synthetic epitopes to induce antigen-specific IgG responses and partial to full protection following viral challenge.
Despite these promising findings, no candidate has reached Phase I or II clinical trials, either in humans or in large-scale field settings under GMP conditions. Available data from preclinical experiments generally report on immunogenicity, safety in animal hosts, and preliminary protection outcomes, but lack standardized regulatory metrics that would support clinical trial authorization. Additionally, many formulations studied—such as those expressed in N. benthamiana, alfalfa, or maize—have not yet undergone scale-up or formal toxicological assessment in compliance with regulatory frameworks.
Several studies highlight the need for further validation through well-designed pilot trials in target species under field conditions, potentially under the supervision of national veterinary authorities. As of the time of this synthesis, there is no evidence of clinical evaluation of plant-based FMD vaccine candidates registered in international trial databases or reported in peer-reviewed literature.
Economic modeling of plant-based vs. conventional FMD vaccines: A limited number of studies have attempted to quantify the economic advantages of plant-based recombinant vaccines over conventional inactivated FMD vaccines. Existing models suggest that, once scaled, plant-based systems may offer competitive cost structures, particularly in regions where cold-chain logistics and high biosafety containment add substantial cost to traditional vaccine manufacturing 7,32.
Preliminary techno-economic assessments estimate that plant expression platforms—especially Nicotiana benthamiana transient expression systems—can produce recombinant antigens at a cost of $0.30–$0.90 per dose, depending on yield efficiency, formulation requirements, and downstream purification methods 27. In contrast, conventional inactivated FMD vaccines typically require BSL-3 facilities and can incur costs of $1.20–$2.50 per dose when factoring in virus propagation, inactivation, adjuvant addition, and cold-chain delivery 4,21.
A key economic differentiator is facility infrastructure. Plant-based systems do not require the same level of biocontainment since no live virus is handled, potentially reducing capital expenditure by over 50% in facility setup 23. Furthermore, modular plant production units can be rapidly deployed in endemic regions, enabling local vaccine production with lower dependency on global supply chains 28.
However, the absence of established GMP pipelines for plant-based vaccine production, combined with regulatory ambiguity and potential IP licensing costs, may counterbalance early cost benefits 9,31. Moreover, conventional vaccines benefit from established procurement and distribution channels, government subsidies, and validated efficacy records, which may complicate market entry for new plant-derived products.
Taken together, available economic modeling suggests that while plant-based FMD vaccines hold promise for cost-effective production and distribution, particularly in Low-and Middle-Income Countries (LMICs), realization of these benefits will depend on resolving upstream regulatory and IP challenges, ensuring batch-to-batch consistency, and achieving adequate production yields in scalable platforms (Table 4).
Discussion :
The development of plant-based recombinant vaccines against FMDV marks a significant advancement in biotechnology, effectively bridging the fields of plant molecular farming and veterinary immunology. This innovative approach leverages the unique advantages of plants as biofactories—such as cost-effectiveness, scalability, and safety—while addressing the urgent global need for effective and accessible FMD vaccines, particularly in regions where conventional vaccine production is constrained. A comprehensive synthesis of recent research 6,7,33 elucidates both remarkable technical progress and persistent challenges that continue to shape this evolving field.
Central to the success of plant-based FMD vaccines is the selection of the appropriate plant host and expression strategy. Among the various platforms, transient expression systems—especially in N. benthamiana—have emerged as a favored method due to their rapid turnaround, flexibility, and relatively high protein yields 8,11. Agroinfiltration enables the introduction of viral vectors or gene constructs into plant leaves, resulting in robust expression of immunogenic FMDV proteins such as VP1 or the assembly of VLPs 9. These VLPs closely mimic the native virus structure, enhancing immune recognition without the risk of infection.
Conversely, stable transgenic plants like alfalfa 30 and maize 25 provide promising platforms for oral vaccine delivery, leveraging the edible nature of these crops to facilitate mucosal immunization. However, stable systems often face bottlenecks such as lower expression levels and antigen degradation during plant growth or processing, which can compromise vaccine efficacy 29,30. To overcome these limitations, molecular engineering strategies have been deployed, including the design of fusion proteins where the FMDV VP1 antigen is linked to carriers such as the CTB or the hepatitis B core antigen. These fusions enhance antigen stability and target mucosal immune pathways by promoting uptake through gut-associated lymphoid tissue, thereby eliciting stronger and more specific immune responses 28,32.
The immunization route profoundly influences the quality and breadth of the immune response generated by plant-based vaccines. Oral delivery is highly advantageous for its non-invasive nature, potential for mass vaccination, and capacity to induce mucosal immunity—the first line of defense against respiratory and oral viral infections such as FMDV 12,13. Nevertheless, oral vaccines often face challenges including antigen degradation in the gastrointestinal tract and induction of oral tolerance, which can dampen immune responsiveness. To address this, co-administration of mucosal adjuvants or the use of fusion constructs that target antigen delivery to mucosal surfaces has been shown to enhance immune activation 20,30.
Alternatively, SC injections consistently generate robust systemic IgG responses and have been extensively employed in transient expression studies to demonstrate vaccine efficacy 1,10. Notably, prime-boost vaccination regimens that combine mucosal priming with systemic boosting are emerging as a promising strategy to induce a balanced and durable humoral and cellular immune response, leveraging the advantages of both delivery routes 14.
Previous studies have demonstrated the potential for robust immune responses, such as high IgG titers and varying rates of protection, in animal models following administration of plant-derived antigens 10,28. For instance, Huang et al 28 reported significant induction of IgG antibodies in animals vaccinated with epitope–HBc fusion proteins expressed in transgenic tobacco, yet comparative protection rates across studies differ substantially due to heterogeneity in experimental design and reporting practices. Kenubih et al 4 highlights that long-lasting immunity remains an ongoing challenge, with observed antibody responses often lacking correlation with actual protection in field trials. Similarly, Alsharifi et al 6 and Chinsangaram et al 25 have reported promising developments with plant-produced virus-like particles and VP1 antigens, respectively, but emphasize the variability of immune readouts attributed to inconsistent methodologies. The variability in assay types, time-points for serum collection, and reporting units, as well as the common omission of statistical measures such as standard deviations and group sizes, precludes the implementation of a meta-analytic approach in this domain. To illustrate, published IgG titers after immunization range from approximately 1:800 to over 1:32,000 28, but direct pooling of such data requires access to primary statistics that are seldom fully reported. Similarly, reported protection rates following challenge vary from 60 to over 90%, contingent upon the antigen construct and host species 4,6,25, but lack of uniform control groups and inconsistent challenge protocols makes data aggregation difficult.
Cost-effectiveness is a pivotal factor in the adoption and large-scale implementation of FMD vaccines, especially in resource-limited settings. Plant-based recombinant vaccines offer several potential economic advantages compared to conventional vaccines produced in cell culture or animal systems. Firstly, the cost of upstream production in plants is markedly lower due to the absence of expensive fermenters, reduced need for cold chain logistics during plant cultivation, and lower biosafety risks 4. Infrastructure requirements for greenhouse or open-field cultivation can be more easily scaled in endemic regions, further reducing per-unit production costs 3. In addition to the lower production expenses, plant expression systems minimize downstream purification costs, as many platforms enable fusion to carrier proteins or facilitate direct oral administration which reduces processing steps 6. By contrast, the manufacture of conventional FMD vaccines entails significant costs for antigen propagation in large bioreactors, multiple rounds of purification, viral inactivation, and quality control to ensure safety—each process contributing to higher final product prices and frequent production bottlenecks 7. Some studies estimate that plant-based FMD vaccines could ultimately reduce final product costs by 30–50% relative to conventional vaccines—provided that large-scale yields and streamlined regulatory pathways are achieved 6,7.
Despite encouraging immunogenicity outcomes, transitioning from laboratory to large-scale production remains a formidable challenge. Low antigen yield per plant biomass, batch-to-batch variability, and the complexity of downstream processing—especially for assembling and purifying VLPs—pose significant obstacles to cost-effective manufacture 7,9. Moreover, post-translational modifications such as glycosylation, which are critical for proper antigen folding and immunogenicity, differ markedly between plants and mammalian systems, potentially influencing vaccine potency and safety 24. Optimizing transient expression conditions for agricultural scalability and developing robust purification protocols remain essential goals 12.
IP rights and the patent landscape play a crucial role in shaping the path toward commercialization of plant-based recombinant vaccines for FMD. The development, production, and global deployment of novel vaccines are often influenced by the presence or absence of broad patents covering vaccine components, production platforms, or adjuvant technologies. In particular, patents on VLP technology, epitope design, or specific plant expression systems may create barriers to entry or require complex licensing agreements for developers and manufacturers aiming to introduce new FMD vaccines to the market 27. Moreover, the fragmented nature of current IP portfolios, often spanning multiple jurisdictions and involving both public and private developers, can further complicate technology transfer and distribution efforts, especially in low- and middle-income countries. Recent advances in synthetic biology and recombinant protein engineering for FMD vaccine design have led to an increase in the number of patent filings related to multi-epitope constructs and scalable manufacturing methods 9,27. However, the majority of patents in the field are concentrated in a limited number of countries and institutions, potentially restricting access and slowing the widespread adoption of innovative plant-based vaccine platforms. Strategic collaboration between patent holders, open-access initiatives, and the development of non-proprietary or patent-expired technologies may be essential for facilitating broader commercialization, particularly in regions where FMD remains endemic 31.
To date, no plant-produced FMD vaccine has achieved regulatory approval, reflecting significant gaps in the oversight of GMOs and veterinary biologics derived from plant platforms 15,33. Key regulatory challenges include establishing vaccine safety and efficacy in target species, demonstrating DIVA capabilities essential for disease surveillance, and meeting GMP standards adapted for plant-based bioproduction. Additionally, regulatory harmonization across countries—especially those endemic for FMD—is urgently needed to facilitate international trade and vaccine deployment 6.
To date, the majority of research on plant-based recombinant vaccines for FMD has remained at the preclinical stage, with studies primarily conducted in animal models 22. While these investigations have demonstrated that plant-derived vaccine candidates can induce robust immune responses and confer protection against FMDV in livestock, no plant-based FMD vaccine has yet advanced to clinical trials involving human participants. This gap reflects the early stage of development and the technical, regulatory, and economic challenges that must be addressed before progressing to human studies 15.
Recent reviews consistently highlight that there are currently no published, peer-reviewed reports of Phase I or Phase II clinical trials for plant-based FMD vaccines. The available literature emphasizes the need for further optimization, larger-scale production, and additional safety and efficacy data from animal trials before clinical evaluation can be considered. As a result, most discussions about clinical potential remain speculative, with future progress dependent on overcoming current scientific and regulatory barriers 19.
Several methodological inconsistencies were identified across the studies included in this meta-synthesis:
1. Lack of standardized assays to evaluate vaccine efficacy, resulting in variability in outcome measures.
2. Missing or incomplete statistical data, such as Standard Deviations (SDs) and sample sizes, limiting the ability to perform robust meta-analyses.
3. Variations in animal models used (e.g., species, age, health status) and challenge protocols (e.g., virus strains, inoculation doses, routes), which may affect comparability of results and interpretation of vaccine performance.
Conclusion :
Plant-based recombinant vaccines against FMD represent a transformative approach that combines advances in plant molecular farming with the urgent need for safer, cost-effective, and scalable veterinary vaccines. The predominance of transient expression systems in N. benthamiana has accelerated vaccine development by enabling rapid production of immunogenic antigens such as VP1 and VLP. Oral immunization strategies leveraging edible plants show promise for inducing mucosal immunity, though challenges remain in antigen stability and delivery. Despite notable progress in immunogenicity and preclinical efficacy, significant obstacles persist in scaling up production, ensuring consistent antigen quality, and navigating an evolving regulatory landscape. Addressing these barriers through enhanced antigen design, optimized expression platforms, and harmonized regulatory frameworks will be critical to translating plant-based FMD vaccines from laboratory research to field application. Ultimately, these innovations hold considerable potential to contribute substantially to global FMD control and livestock health worldwide.
Harmonization of global regulatory frameworks is essential to expedite the development, evaluation, and commercialization of plant-based recombinant FMD vaccines. International collaboration among regulatory agencies, standard-setting bodies, and research institutions can help establish clear guidelines for quality assurance, safety assessment, and licensing of novel vaccine platforms. Policymakers should prioritize building transparent and science-based approval processes that facilitate technology transfer, streamline cross-border trade, and foster public–private partnerships. Adoption of mutual recognition mechanisms and the creation of unified GMP standards for plant molecular farming would greatly reduce duplication of regulatory efforts and accelerate access to safe and affordable vaccines in FMD-endemic regions.
Conflict of Interest :
The authors declared no conflict of interest.
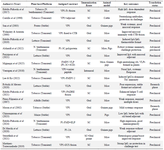
Table 1. Overview of Selected Studies on Plant-Based Recombinant Vaccines against FMD (2000–2025)
Cholera Toxin B (CTB), Virus-Like Particles (VLPs), Subcutaneous (SC).
|
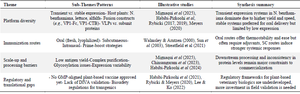
Table 2. Thematic synthesis of plant-based recombinant FMD vaccine research (2000–2025)
Differentiating Infected from Vaccinated Animals (DIVA): Virus-Like Particles (VLPs).
|
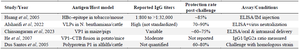
Table 3. Immune response profiles and protection rates in preclinical studies of plant-based FMD vaccines
Cholera Toxin B (CTB).
|
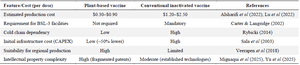
Table 4. Comparative economic modeling of plant-based vs. conventional FMD vaccines
|
|