Swimming Exercise Attenuates DOX-Induced Cardiotoxicity by Modulating Apoptosis and DRP1/PGC1α/ miR-23a Dependent Pathway in Rat Heart Tissue
-
Darvakh, Hassan
-
Shohadayehoveizeh Campus of Technology, Shahid Chamran University of Ahvaz, Dashteazadegan, Iran
-
 Shakerian, Saied
Department of Sport Physiology, Faculty of Sport Science, Shahid Chamran University of Ahvaz, Ahvaz, Iran, Tel: +98 9163143363; E-mail: s.shakeryan@scu.ac.ir
Shakerian, Saied
Department of Sport Physiology, Faculty of Sport Science, Shahid Chamran University of Ahvaz, Ahvaz, Iran, Tel: +98 9163143363; E-mail: s.shakeryan@scu.ac.ir
-
Ranjbar, Rohollah
-
Department of Sport Physiology, Faculty of Sport Science, Shahid Chamran University of Ahvaz, Ahvaz, Iran
-
Tabandeh , Mohammad reza
-
Department of Basic, Science, Division of Biochemistry and Molecular Biology, Faculty of Veterinary Medicine, Shahid Chamran University of Ahvaz, Ahvaz, Iran
Abstract: Background: Doxorubicin (DOX) is a widely used drug in cancer chemotherapy, but its cardiotoxicity limits its clinical applications. Combining exercise with chemotherapy offers a promising approach to mitigate the side effects of chemotherapy drugs. Limited information is available on the effects of swimming exercise on the molecular mechanisms related to Dox cardiotoxicity. This study aims to investigate the modulatory effect of swimming exercise on the apoptosis and miR-23a-dependent mitochondrial biogenesis and dynamics pathways in rat heart tissue treated with dox.
Methods: In this experimental study, thirty-two adult male Wistar desert rats (200-220 g) were randomly divided into four groups, including control, doxorubicin (DOX; intraperitoneal injection of 5 mg/kg of Dox, once a week, for five weeks), swimming exercise (SE; water exercise for 60 min/day, five days a week, for six weeks) and Dox group along with Swimming Exercise (DOX-SE). At the end of the study, the cardiac expression of proteins related to apoptosis and mitochondrial biogenesis and mir23-a were analyzed using western blot and real-time PCR methods, respectively. One-way analysis of variance (ANOVA)with Tukey's post hoc test was used to analyze the data.
Results: These findings revealed that DOX administration led to a significant decrease in the cardiac expression of PGC-1α and DRP-1 proteins and an increase in apoptotic proteins (caspase 3 and cytochrome C) compared to the control group (p<0.0001). Swimming exercise resulted in a significant increase expression in cardiac tissue of PGC-1α and DRP-1 proteins and a decrease in the expression of apoptotic proteins in the DOX-treated group (p<0.0001, p<0.01). Compared to the control group, the protein levels in the heart of the miR-23a were significantly increased in the DOX-treated group (p<0.001). However, exercise training attenuated the DOX-induced reduction in miR-23a expression gene in the cardiac muscle of DOX-treated mice (p<0.05).
Conclusion: These findings suggest that swimming exercise may protect against DOX-induced cardiotoxicity by regulating apoptosis and DRP1/PGC1α/ miR-23a pathway. This highlights exercise as a potential non-pharmacological strategy to mitigate chemotherapy-related heart damage.
Introduction :
Doxorubicin (DOX) belongs to the family of anthracyclines, which is used in the treatment of a wide range of malignancies. Cardiotoxicity is one the most important side effects of DOX, which poses a significant challenge in the successful use of this drug for cancer treatment 1. Cardiac side effects of DOX occur through various mechanisms, including changes in cellular metabolic processes, apoptosis, autophagy, oxidative stress, and mitochondrial dysfunction 2. DOX induces programmed cell death (apoptosis) in cardiomyocytes by the release of cytochrome c (CytC) from mitochondria, activation of caspases, and subsequent degradation of cellular proteins, ultimately resulting in the loss of functional heart cells. Furthermore, the activation of apoptotic pathways is closely linked to mitochondrial dysfunction, as DOX-induced apoptosis often disrupts mitochondrial membrane integrity, compromising ATP production and exacerbating mitochondrial damage. Alterations of these pathways result in symptoms of heart failure such as reduced exercise tolerance, shortness of breath, and fluid retention 3.
Mitochondrial biogenesis involves the dynamic processes of mitochondrial fission and fusion, which are crucial for maintaining mitochondrial function and health 4. Mitochondrial fission is crucial for maintaining mitochondrial morphology, distribution, and function within cells. Drp1 is a key regulator of mitochondrial fission process. Excessive activation of Drp1 following DOX treatment led to mitochondrial fragmentation and dysfunction, triggering apoptotic pathways, increasing reactive oxygen species production, and promoting inflammatory responses in cardiomyocytes 5. Drp1-mediated mitochondrial fission facilitates the release of Cytc from the mitochondria, which then activates the caspase cascade and ultimately leads to cell death 6. PGC-1α is a central regulator of mitochondrial function in the cardiac muscle, promoting mitochondrial biogenesis, optimizing energy metabolism, enhancing mitochondrial respiratory capacity, and maintaining mitochondrial quality and integrity. Dysregulation of PGC-1α activity has been implicated in various cardiac diseases, highlighting its importance in maintaining cardiac mitochondrial function and overall cardiac health 7,8. Previous studies have shown that PGC-1α expression is reduced in DOX-induced cardiomyopathy 9. A recent study showed that PGC-1α was decreased in the hearts of DOX-treated mice and H9c2 cells 10,11.
MicroRNAs (miRNAs) are small non-coding RNA molecules with a length of 21-32 nucleotides, which often reduce the gene expression by connecting to the UTR-3 region of the target RNA, leading to their degradation or translational repression 12. It has been reported that miRNAs play a fundamental role in cardiotoxicity induced by DOX and some circulating miRNAs has been determined as potential biomarkers of DOX-induced cardiotoxicity 13. Previous studies have shown that PGC-1α is a direct target of miR-23a 9. Du et al indicated that inhibition of miR-23a attenuates DOX-induced mitochondria-dependent cardiomyocyte apoptosis and improve mitochondrial biogenesis by targeting the PGC-1α/Drp1 pathway. Li et al, found that treatment with DOX enhances the expression of miR-23a and triggers caspase-3 activation in cardiomyocytes. In addition, inhibiting the transcription of miR-23a decreased apoptosis induced by DOX by interacting with p53 and enhancing its binding to the miR-128 promoter. These results indicate that miR-23a functions as a pro-apoptotic microRNA in cardiomyocytes exposed to DOX 14.
Exercise has been proposed as a potential therapeutic strategy to mitigate the cardiotoxic effects of DOX. Various types of exercise, including aerobic and resistance training, have been shown to offer protective effects against chemotherapy-induced cardiotoxicity by modulating several cellular and molecular pathways. Several studies have shown that exercise training or physical activity prevents or reduces the cardiac dysfunction caused by DOX. Also, exercise inhibits both intrinsic and extrinsic apoptotic pathways in DOX-treated animals. It has been reported that exercise preconditioning inhibits the expression of caspase-9 and caspase-3 proteins in DOX-treated animals, indicating that exercise reduces the intrinsic apoptotic pathway induced by DOX 15.
Despite the potential benefits of exercise in protecting against chemotherapy-induced cardiotoxicity, limited information is available on the effects of swimming exercise on the molecular mechanisms related to DOX cardiotoxicity, particularly focusing on apoptosis and miR-23a-dependent mitochondrial biogenesis and dynamics pathways. Therefore, this study aims to investigate the modulatory effect of swimming exercise on the expression of proteins related to apoptosis and miR-23a-dependent mitochondrial biogenesis and dynamics pathways in rat heart tissue treated with DOX.
Materials and Methods :
Animals and groups: This study was registered under the code: IR.SCU.REC.1402.068 in Research Ethics Committee of Shahid Chamran University of Ahvaz. In this Experimental study, 32 adult male Wistar rats (12 weeks old, 200-220 g) were obtained from the animal center of Shahid Chamran University of Ahvaz, Ahvaz, Iran. The rats were maintained in a regulated environment with a temperature of 25±2°C, humidity levels of 55±15%, and a 12-hr light/dark cycle. They were provided unlimited access to water and standard rodent feed from Parsfeed Co., Iran. Before the start of experiments, the animals were allowed a two-week period to acclimate to these laboratory conditions. All experimental protocols were approved by the Ethics Committee guidelines of Shahid Chamran University of Ahvaz (IR.SCU.REC.1402.068). The animals were randomly divided into four groups including Control or negative control (C), DOX (D) (positive control), Swimming exercise (S) and DOX+Swimming exercise (DS). The control group received normal saline, and the treatment groups received DOX as an intraperitoneal injection at 4 mg/kg once a week for five weeks 16. Injections were given every week after 24 hr after the last weekly training session (Figure 1).
Swimming exercise protocol: Swimming training was done in a glass container with a length of 100 cm, a width of 50 cm, and a height of 70 cm, up to a height of 40 cm with a water temperature of 35°C. The water activity training program was held in two stages: education and training. The training phase lasted one week. On the first day, rats performed a 10-min swimming session in a standard tank (100×50×70 cm) filled with water at 35°C under turbulent conditions. The duration of swimming increased by 10 min each day, reaching 60 min by the end of the week, allowing the rats to progressively adapt to the exercise regimen. Following this acclimatization, the exercise phase was conducted for 60 min/day, five days per week, over a period of six weeks 17.
Sampling: At the end of the experiment, the rats were anesthetized using ketamine hydrochloride (100 mg/kg) and xylazine (10 mg/kg), and blood samples were collected by heart puncture. Then, the heart tissue was separated and stored in a -80°C freezer.
Western blot analysis Western blot analysis was used to determine the expression levels of caspase-3, DRP-1 proteins. Briefly, the tissue samples were homogenized in 250 μl of RIPA lysis buffer (containing 150 mM NaCl, 0.1% SDS, 25 mM Tris at pH=7.4, 1 mM NaF, 1 mM phenylmethylsulfonyl fluoride, 50 mM sodium fluoride, and a protease inhibitor cocktail from Sigma, USA) using a homogenizer (Heidolph, Germany). The homogenized tissues were then centrifuged at 10,000 RPM for 15 min at 4°C. After determining protein concentration in tissue lysates with Bradford assay, 20 µg of samples were loaded onto a 10% Sodium Dodecyl Sulfate-Polyacrylamide (SDS-PAGE) gel and separated by electrophoresis. Finally, proteins were transferred to polyvinylidene difluoride membranes (Amersham, USA; 10600023). Membranes were blocked with 5% non-fat dry milk (Merck, Germany) and then with primary antibodies: anti-DRP-1 mAb (#8570, Cell Signaling Technology, USA), anti-CASPASE -3 antibody (#9662, Cell Signaling Technology, USA), anti-PGC1 antibody (SL7534R‚SunLong Biotech ‚China), and GAPDH (#2118, Cell Signaling Technology, USA) were probed at 4°C. The membrane was washed with Tris-phosphate buffer saline (TPBS; PBS and 0.1% Tween 20), incubated further with secondary antibody anti-rabbit IgG (#7077, Cell Signaling Technology, USA) at room temperature, and washed with TPBS. After three washes, protein reactivity was visualized using an ECL detection kit (Pars Tous, Iran; B111420). The blots were detected using the Fusion X chemiluminescence imaging system (Vilber, USA). Optical Density (OD) analysis was performed using the Fusion X software and expressed as fold change the control group.
Measurement of tissue CYTC concentration: Tissue Cytc levels were measured in tissue homogenate using a rat-specific ELISA kit according to the manufacturer’s instructions (Sunlong Biotech‚ China; SL1686Ra), and the results were expressed as pg/mg protein.
Evaluation of cardiac mir23-a expression: The expression level of miR-23a was evaluated using quantitative real-time PCR assay. The miRNA was extracted using minute miRNA Isolation Kit (TIANGEN‚ China; 4992860) as recommended by the manufacturer’s protocol. The purity of the miRNA was assessed at a 260/280 OD ratio with an Eppendorf µCuvette G1.0 microvolume measuring cell (Eppendorf, Germany). High-purity miRNA with an OD of 260/280 ratio above 1.8 was used for cDNA synthesis. The cDNA was synthesized using the miRcute miRNA First-strand cDNA Synthesis Kit (TIANGEN‚ China; 4992786) based on the polyadenylation method as suggested by the manufacturer’s instruction. Quantitative real-time PCR (qRT-PCR) was conducted using the SYBR® Green Real-Time PCR Master Kit (ParsTous, Iran; C101022) on the StepOnePlus™ Real-Time PCR Detection System (Applied Biosystems, USA). The primers were designed using sRNAPrimerDB online software (http://www.srnaprimerdb.com).
The primer sequences are shown in table 1. The thermal cycling program included an initial denaturation at 95°C for 5 min, followed by 45 cycles of 94°C for 15 s and 60°C for 25 s. All experiments were performed in triplicate. Additionally, two separate reactions without cDNA or miRNA were run in parallel as negative controls. Relative quantification was performed according to the comparative 2-∆∆Ct method, using StepOne software 2.3. U6 was used as a housekeeping gene. Validation of assay to check whether the primers of target genes and housekeeping gene had similar amplification efficiencies were performed as described previously 18.
Statistical analysis: Statistical analyses and graph design were conducted using GraphPad Prism software version 9 (GraphPad Software, Inc., San Diego, CA). To evaluate the statistical significance of differences between groups, a one-way analysis of variance (ANOVA) followed by Tukey's post hoc test was employed. Data are presented as mean±SD. Statistically significant differences between experimental groups are indicated as follows:
* p<0.05, ** p<0.01, *** p<0.001, and **** p<0.0001.
Results :
Effect of water exercise on the cardiac expression of drp1 and PGC1-α: Treatment of rats with DOX led to a significant decrease in PGC-1 protein expression compared to the control group (p<0.0001). The protein expression of PGC-1 in the swimming+DOX group was significantly higher than that of the DOX group (p<0.0001). Swimming exercise alone led to a significant increase in the expression of PGC-1 compared to the control group (p<0.05) (Figure 2).
The level of DRP1 protein expression in rats with swimming exercise was not significantly different compared to the control group. The level of DRP1 protein expression in the DOX-treated group was significantly decreased compared to the control group (p<0.0001). Swimming exercise could up regulate the expression of DRP1 protein in the heart of DOX treated group when compared to DOX treated group without exercise (p<0.01) (Figure 3).
The level of Caspase3 protein expression in the group of rats with Swimming exercise was not significantly different from the control group. DOX treatment resulted in an elevation of caspase3 protein expression compared to the control group (p<0.0001). It was observed that the expression of Caspase3 protein in the DOX+swimming group was significantly reduced compared to the group rats that were treated only with DOX (p<0.001) (Figure 4).
The Cytc levels in the heart of rats with swimming exercise were not significantly different compared to the control group. The cardiac level of Cytc in the DOX group was significantly increased compared to the control group (p<0.0001). Swimming exercise in DOX rats could reduce the Cytc levels in heart of rats treated with DOX compared to the group of rats that were treated with only DOX (p<0.01) (Figure 5).
The expression levels of miR-23a in the group of rats with swimming exercise was not significantly different compared to the control group. In the group treated with DOX, there was a significant increase in the expression of the miR-23a gene compared to the control group (p<0.001). Also, a significant decrease was observed in the expression of the miR-23a gene in the rats treated with DOX and swimming exercise (D-S) compared to those treated with DOX alone (p<0.05) (Figure 6).
Discussion :
DOX induces mitochondrial dysfunction, which is critical in the high-energy-demanding heart. The cardiotoxicity induced by DOX involves mitochondrial dynamic imbalance, mitochondrial dysfunction, activation of apoptotic pathways, DNA damage, and oxidative stress. This study investigated the effect of swimming exercise on apoptosis, mitochondrial biogenesis, and dynamics-related proteins regulated by miR-23a in the cardiac muscle tissue of DOX-treated rats 19.
The study findings indicated that six weeks water exercise in DOX treated rats led to an increased expression of genes involved in mitochondrial biogenesis (DRP1 and PGC1) and decreased expression of apoptotic genes (Caspase 3 and CYTC).
Under normal physiological conditions, the number, morphology, and function of mitochondria are regulated to maintain cardiac homeostasis 20. The Mitochondrial Quality Control (MQC) system, which includes the removal of damaged mitochondria, generation of new mitochondria by biogenesis, isolation of damaged mitochondria by fission, and exchange of mitochondrial content by fusion, is essential for maintaining a healthy and functional mitochondrial network 21. Alteration in these mitochondrial processes can lead to mitochondrial dysfunction. PGC-1α, as a master regulator of mitochondrial biogenesis, coordinates genes involved in energy metabolism and modulates mitochondrial dynamics 22. The study findings indicated that six weeks of swimming exercise in DOX-treated rats led to increased expression of PGC-1α and DRP1 proteins, which are crucial for mitochondrial biogenesis and fission, respectively.
Recent studies have demonstrated that PGC-1α directly interacts with the DRP1 gene promoter to modulate DRP1 transcription levels, which influences mitochondrial fission 22. A study by Sui et al has shown that the positive regulation of PGC-1α can increase the expression of mitochondrial fusion genes such as MFN2 and OPA1 and decrease the expression of mitochondrial fission genes such as DRP1 and FIS1 23. Consistent with the results of the present study, previous studies have shown that DOX induces excessive mitochondrial fission characterized by upregulation of DRP1 phosphorylation and downregulation of mitofusin 2 (Mfn2) and mitochondrial-dependent apoptosis in cardiomyocytes 2. Previous studies have also shown that PGC-1α is inhibited in DOX-induced cardiomyopathy 24. The increased expression of PGC-1α and DRP1 in this study suggests a positive effect of swimming exercise in enhancing mitochondrial biogenesis and maintaining mitochondrial dynamics in DOX-treated hearts.
The findings of the present study revealed that the expression of caspase 3 and the tissue level of CYTC were elevated in the DOX treated rats compared to the control group. However, swimming exercise reversed these changes in the heart tissue of DOX-treated rats 25. CYTC plays a crucial role in mitochondrial damage. Increased permeability of the damaged mitochondrial membrane leads to the leakage of cytochrome c from the mitochondria and finally leads to cardiomyocyte apoptosis 26. CYTC by binding to Apoptotic Protease Activating Factor-1 (Apaf-1) activates procaspase-9 and caspase-9 as the caspase that initiates apoptosis in the mitochondrial pathway. This process finally activates caspase-3 as an executive caspase and the interface of all apoptotic pathways 27. By reducing the levels of CYTC and caspase-3, swimming exercise mitigated the apoptotic response in DOX-treated rats, suggesting its potential as a non-pharmacological intervention to reduce cardiotoxicity.
The protective effect of swimming exercise against apoptosis is consistent with research showing that exercise can reduce apoptotic signaling in cardiomyocytes. Swimming exercise-induced enhancement of mitochondrial biogenesis and function, through increased PGC-1α and DRP1 expression, likely contributes to the stabilization of mitochondrial membranes and reduction in cytochrome c release. The release of cytochrome c as a pro-apoptotic factor facilitated by Mitochondrial Outer Membrane Permeabilization (MOMP), a process regulated by Bcl-2 family proteins. Enhanced PGC-1α and DRP1 expression leads to healthier mitochondria that are less prone to MOMP and subsequent cytochrome c release. This stabilization prevents the activation of the apoptotic cascade, which is initiated by CYTC binding to Apaf-1 and the formation of the apoptosome, leading to caspase-9 and caspase-3 activation. These mechanisms highlight the potential of swimming exercise as a therapeutic strategy to mitigate cardiotoxicity by promoting mitochondrial biogenesis and reducing cell death 28.
The present study showed that the expression of mir-23a was increased in heart of DOX treated rats, while swimming exercise could attenuate the increased expression of mir-23a in DOX treated animals. Recent research indicated the regulatory role of miR32-a on the expression of genes related to mitochondrial biogenesis 29. These findings may contribute to the observed increase in PGC-1α and DRP1 and the reduction in apoptotic markers. The downregulation of miR-23a by swimming exercise suggests that exercise may mitigate DOX-induced cardiac damage by altering miRNA expression profiles. To confirm this opinion previous findings Long et al has shown that elevation of mir-23a is crucial in cardiac apoptosis caused by ischemia. Previous studies have also shown that PGC-1α is a direct target of mir-23 in estrogen deficiency-induced concentric remodeling 9, Du et al investigated the effect of mir-23a by targeting the DRP-1 PGC-1α pathway on DOX-dependent mitochondrial apoptosis in the left ventricle of the rat heart, and the results showed that the increase of mir-23a caused mitochondrial damage in this cardiac cell toxicity model 9. The inhibiting mir-23a expression can also reduce the cardiac damage and inhibits mitochondria-dependent apoptosis by directly targeting the DRP1/PGC-1α pathway 9. These findings indicate that suppression of miR-23a transcription by swimming exercise may attenuate the inhibitory effects of miRNA-23a on PGC-1α and DRP1, positively regulating their expression and thus improving mitochondrial function in heart of DOX treated animals.
Despite these promising findings, this study has limitations that warrant further investigation. The study was conducted on a specific strain of rats, which might limit the generalizability of the findings to other species or even other rat strains. Further studies are needed to validate findings across different models. We utilized a fixed dose of DOX and a specific regimen of swimming exercise. Variations in dosage and exercise intensity could yield different outcomes, which we did not explore in this study. Also, this study focused on the immediate impacts of swimming exercise on DOX-induced cardiotoxicity. Long-term studies are necessary to understand the durability of these protective effects. While we have discussed potential molecular pathways influenced by swimming exercise, direct causal relationships between these pathways and the observed phenotypic changes were not established. Future studies should aim to elucidate these mechanisms more clearly.
Conclusion :
The present study demonstrated that water-based exercise can effectively downregulated miR-23a expression and concomitantly upregulate the expression of PGC-1α and DRP1 the heart of rats treated with DOX. Additionally, water exercise was found to suppresses apoptosis by downregulating caspase3 and inhibiting the release of CYTC. These findings suggest that water exercise may offer a promising non-pharmacological approach to mitigate DOX-induced cardiotoxicity by enhancing mitochondrial biogenesis, maintaining mitochondrial dynamics, and suppressing apoptotic pathways in the heart. Further studies are warranted to elucidate the underlying mechanisms and to explore the potential clinical applications of water exercise interventions in the management of chemotherapy-induced cardiotoxicity.
Ethical approval :
This study was approved by the Research Ethics Committee of Shahid Chamran University of Ahvaz, Iran (as registered under the code: IR.SCU.REC.1402. 068) under the NIH Guidelines for the Care and Use of Laboratory Animals (NIH publication, 1996).
Conflict of Interest :
The authors declare no conflict of interest.
Funding: No funding was received to assist with the preparation of this manuscript.
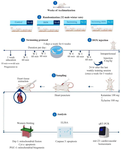
Figure 1. Schematic figure of materials and methods.
|
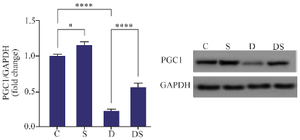
Figure 2. The effect of swimming on the expression of PGC1 protein in the heart of rats treated with Doxorubicin. The results are reported as mean±standard deviation. GAPDH protein was used as a calibrator. All assays were performed in triplicates. * and **** indicate a significant statistical difference between different experimental groups at p<0.05 and p<0.0001, respectively. C (control group), S (swimming group), D (DOX group), DS (swimming + DOX group).
|
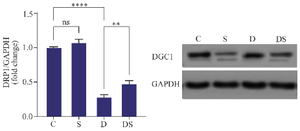
Figure 3. The effect of swimming on the expression of DRP1 in the heart of rats treated with Doxorubicin. The results are reported as mean±standard deviation. GAPDH protein was used as a calibrator. All assays were performed in triplicates. ** and **** indicate the significant statistical difference between different experimental groups at p<0.01 and p<0.0001, respectively. (C (control group), S (swimming group), D (DOX group), DS (swimming + DOX group).
|
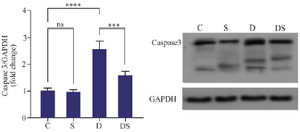
Figure 4. The effect of swimming on the expression of Caspase3 in the hearts of rats treated with Doxorubicin. The results are reported as mean±standard deviation. GAPDH protein was used as a calibrator. All assays were performed in triplicates. *** and **** indicate a significant statistical difference between different experimental groups at p<0.001 and p<0.0001, respectively. C (control group), S (swimming group), D (DOX group), DS (swimming + DOX group).
|
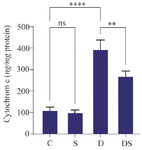
Figure 5. The effect of swimming on the protein level of cytochrome C in the heart of rats treated with DOX. The results are reported as mean±standard deviation. All assays were performed in triplicates. ** and **** indicate a significant statistical difference between different experimental groups at p<0.01 and p<0.0001, respectively. C (control group), S (swimming group), D (DOX group), DS (swimming + DOX group).
|
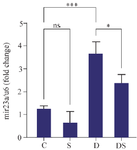
Figure 6. The effect of swimming on the miR-23a gene expression in the heart of rats treated with DOX. The results are reported as mean ± standard deviation. All assays were performed in triplicates. U6 was used as a housekeeping gene. * and *** indicate a significant statistical difference between different experimental groups at p<0.51 and p<0.001. C (control group), S (swimming group), D (DOX group), DS (swimming + DOX group).
|

Table 1. Sequences of primers that used in the current study
|
|