Ameliorating Potential of Quercetin and Curcumin on Glucose-6-Phosphate Dehydrogenase Expression via miRNAs in Rats with Type 2 Diabetes Mellitus
-
Bagheri, Mahsima
-
International Campus of Shahid Sadoughi University of Medical Sciences, Yazd, Iran
-
Khodarahmi , Ameneh
-
Department of Biochemistry, School of Medicine, Shahid Sadoughi University of Medical Sciences and Health Services, Yazd, Iran
-
Zare Mehrjardi, Fatemeh
-
Department of Physiology, School of Medicine, Shahid Sadoughi University of Medical Sciences and Health Services, Yazd, Iran
-
 Moradi, Ali
Department of Biochemistry, School of Medicine, Shahid Sadoughi University of Medical Sciences and Health Services, Yazd, Iran, Tel: +98 35 38207198; Fax: +98 35 6263893; E-mail: morady2008@gmail.com
Moradi, Ali
Department of Biochemistry, School of Medicine, Shahid Sadoughi University of Medical Sciences and Health Services, Yazd, Iran, Tel: +98 35 38207198; Fax: +98 35 6263893; E-mail: morady2008@gmail.com
Abstract: Background: Type 2 diabetes mellitus (T2DM) is accompanied by a significant risk of oxidative stress. While a link between T2DM and G6PD deficiency has been suggested, their interaction is not precisely understood. Furthermore, emerging evidence suggests an expression association between G6PD and miR-1, miR-122, and miR-206. Given the antioxidant and anti-inflammatory properties of Curcumin (Cur) and Quercetin (Q), This study aimed to assess the effects of curcumin and quercetin on G6PD expression and its correlation with the mentioned microRNA expression in liver, renal, heart, and muscle in rats with T2DM.
Methods: RT-qPCR was employed to determine miR-1, miR-122, miR-206, and G6PD expression.
Results: The findings revealed that curcumin and quercetin treatment elevated G6PD gene expression. Also, the treated groups exhibited down-regulation of miR-1, miR-122, and miR-206 (p<0.05). Furthermore, there was a significant inverse correlation between G6PD and miR-1 in heart, miR-122 in all tissues except renal and miR-206 expression in skeletal muscle and heart (p<0.05).
Conclusion: This study suggests that curcumin and quercetin may prevent the development of T2DM by effectively increasing G6PD expression and reducing miR-1, miR-122, and miR-206 expression.
Introduction :
Driven by both genetic and environmental risk factors, Type 2 diabetes mellitus (T2DM) is a complex multifactorial metabolic syndrome with excessive blood glucose levels resulting from reduced insulin secretion or insulin resistance 1. Under intracellular hyperglycemic conditions, glucose triggers an excessive amount of the production of free radicals through various mechanisms. Autoxidation reaction converts glucose into reactive ketoaldehydes and superoxide anion radicals. Additionally, glucose can generate free radicals via promoting lipid peroxidation of Low-Density Lipoprotein (LDL), promoting the synthesis of Advanced Glycation End products (AGEs), activation of Protein Kinase C (PKC) isoforms, and the hexosamine pathway 2. Each process collectively exacerbates Reactive Oxygen Species (ROS) accumulation, leading to lipid, protein, or DNA damage, irreversible oxidative modifications, and potentially contributing to the development of diabetic complications 3. Oxidative stress further plays a critical role in the progression and pathogenesis of diabetes, as redox modifications of various proteins involved in insulin signaling can impair both insulin production and resistance 4,5.
Under oxidative stress, the entire antioxidant system is crucial for cell survival 6. Among these antioxidant components, Glucose-6-Phosphate Dehydrogenase (G6PD) serves as a vital factor in regulating oxidative stress by producing NADPH, a principal intracellular reductant 7. Research indicates that hyperglycemia leads to Protein Kinase A (PKA) activation through increased cAMP. Once activated, PKA phosphorylates and inhibits G6PD activity. Inhibition of G6PD decreases intracellular NADPH levels and lessens oxidative stress in various tissues such as liver, kidney, and heart. Ultimately, enhanced oxidative stress can result in cell damage and apoptosis 8,9.
Reports of recent studies have demonstrated numerous health benefits that can be attributed to active substances derived from plants (phytochemicals). Compared with synthetic biomaterials, these compounds have lower adverse effects and are readily accessible. The therapeutic application of phytochemicals has shown a considerable potential to diminish the risk of developing various diseases.
Curcumin is a subclass of curcuminoids with notable antioxidant capabilities, which originate from the presence of aromatic hydroxyl groups. Also, it usually chelates metals such as Cu2+, Zn2+, Mn2+, Mg2+, and Fe2+ through β-diketone moiety. These metal–curcumin complexes boost the antioxidant activity of curcumin and quench Fenton reactions 10. Furthermore, clinical findings suggest the key role of curcumin in managing T2DM. Curcumin enhances insulin sensitivity in diabetic mice and obese-diabetic animals with insulin resistance and significantly lowers blood glucose levels by activating the phosphatidylinositol-3-kinase/protein kinase B/glucose transporter 2 (PI3K/Akt/GLUT2) signaling pathway and inhibiting phosphorylation of extracellular signal-regulated kinase 1/2 (ERK1/2) 11. Curcumin inhibits glucose transporter 4 (GLUT4) translocation in adipocytes and hepatocytes by impeding the insulin receptor substrate-1 (IRS)/PI3K/Akt signaling pathway, decreasing glucose uptake. It also reduces in oxidative stress through several mechanisms, such as downregulation of Nuclear Factor-κB (NF-κB) and Tumor Necrosis Factor α (TNF-α) expression (similar to resveratrol, a polyphenolic compound classified as a stilbenoid molecular mechanism), upregulation of expression peroxisome proliferator-activated receptor-γ (PPAR-γ), and B-cell lymphoma-2 (Bcl-2), and activation of Janus kinase 2/signal transducer and activator of transcription 3 (JAK2/STAT3) signaling pathway 12.
Extensive research has been conducted on the family of flavonoids quercetin, (-)-epigallocatechin gallate, hesperetin, naringenin, luteolin, and apigenin, which function as ROS-mediated antidiabetics through various mechanisms, such as glucose transporter, hepatic enzymes, tyrosine kinase inhibitor, 5' AMP-activated Protein Kinase (AMPK), PPAR-γ, and NF-κB. In this study, quercetin, the most abundant flavonoid in human diets, is implicated for its antioxidant effects on DT2M. Quercetin is involved in cell proliferation and apoptosis by inhibiting pro-oxidant enzymes, including xanthine oxidase, cyclooxygenases, and protein kinases. 3'-4'-catechol structure in ring B and hydroxyl group on the 3-position of ring C prevent peroxidation reactions by scavenging reactive oxygen and nitrogen species. Similar to curcumin, quercetin is involved in ROS-producing reactions by chelating trace metals. Additionally, this flavonoid maintains glutathione in its reduced state to protect macrophages against oxidative stress 13.
Quercetin has been shown to positively affect glycemia by increasing insulin sensitivity, reducing glucose production in liver cells, and stimulating glucose uptake in tissues by reducing lipid peroxidation, glucose absorption by GLUT2, and the inhibition of insulin-dependent activation of PI3K 14. Quercetin activates an insulin-independent AMPK pathway by interrupting oxygen consumption by Adenosine Diphosphate (ADP) and promotes the translocation and expression of GLUT4 in isolated mitochondria. This mechanism functions similarly to metformin, a medication used to treat type 2 diabetes 15.
MicroRNAs (miRNAs) are non-coding single-stranded nucleotides (~22) that downregulate the gene expression in various biological processes, such as cell differentiation, cell cycle, and apoptosis. Additionally, dysregulation of miRNAs has been associated with various pathological conditions, including developmental abnormalities, diabetes, and tumorigenesis 16. Studies have shown that miR-1 and miR-206 possess probable complementary sites in the 3′ UTRs of G6PD gene 17. The heart tissue miRNA sequencing has revealed that 40% of its total miRNAs consist of miR-1 18. Previous studies revealed that downregulated miR-1 could elevate the expression of G6PD in cancer cells 19. miR-206 represses the pentose phosphate pathway genes (G6PD, PGD, TKT, and GPD2), decreases NADPH production, and blocks cell proliferation 20. The hyperglycemic condition raises miR-1 and miR-206 expression via SRF and MEK1/2 pathways 21. miR-122 is markedly expressed in the liver and can regulate inflammation, apoptosis, and oxidative stress 22. Studies have shown that G6PD mRNA expression is associated with miR-122. It downregulates G6PD expression by binding to its 3′-UTR region 23. Furthermore, it has been reported that a positive correlation has been reported between miR-122 and insulin resistance 24. In another study, miR-122 expression was elevated in patients with diabetic retinopathy 25.
Extensive research suggests phytochemicals impact microRNA expression to control type 2 diabetes, presenting a potential therapeutic approach. It has been reported that administering 60 mg/kg of curcumin improves insulin signaling by enhancing miR-206 expression in fructose-induced insulin signaling disorders rats 26. Moreover, administration of quercetin and epigallocatechin gallate protects islet cells by regulating the expression levels of miR-16-5p, miR-27a-3p, and miR–96–5p in pancreatic β cells 27.
Isorhamnetin, a quercetin metabolite, modulates glucose uptake and insulin resistance by increasing the expression of AKT2 mRNA and upregulating miR-1 and miR-3163 28. Along with these compounds, there are other herbal substances such as resveratrol, liquiritigenin, kaempferol, saccharin, β-sitosterol, agathisflavone, mastiha, which show some modulatory effects on glucose metabolism-related microRNAs 29. Green tea also suppresses the expression of miRNAs triggered by high-fat conditions. In mice fed a High-Fat Diet (HFD), administration of green tea polyphenols decreases miR-335 expression in adipose tissue, which could act as a connection between inflammation and metabolic dysfunction 30.
Taken together, the diverse impacts of phytochemicals on type 2 diabetes and its associated complications involving microRNAs demand more research to clarify their mechanisms. In the current study, we hypothesized that curcumin and quercetin might positively control hyperglycemia and lower oxidative stress by increasing G6PD expression and decreasing miR-1, miR-122, and miR-206 expression.
Materials and Methods :
Chemicals and reagents: The HFD was sourced from the Royan Institute of Isfahan in Iran. Streptozotocin (STZ) and curcumin and quercetin (Purity [high-performance liquid chromatography] of more than 80%), required for the treatment of animals, were obtained from Sigma Chemical Co. (St. Louis, MO, USA). TRIzol reagent was obtained from Invitrogen (Carlsbad, CA). Pars Azmon Diagnostic Co (Iran) commercial assay kits were applied to determine serum glucose level.
Animals and experimental stages: Every protocol followed the current ethical considerations and national norms and standards of the research ethics committees of the School of Medicine, Shahid Sadougi University of Medical Science of Animal Use [IR.SSU.MEDICINE.REC.1400.151]. The interventional-experimental work was conducted on 40 adult male Wistar rats (180–220 g, provided by Pasteur Institute of Tehran, Iran). They were kept in a regular 12h /12h light/dark cycle at 22–24°C with free access to water and food. After one-week adaptation, the animals were randomized into two groups by feeding a normal pellet diet or HFD (58% fat, 25% protein, and 17% carbohydrate, as a percentage of total kcal) for four weeks. Further, the HFD group was fasting overnight and injected once with IP streptozotocin at a dose as low as 35 mg/kg BW in 0.1 mmol/L cold citrate buffer (pH=4.5). In contrast, the related control animals received only citrate buffer. The rats who showed a non-fasting blood glucose level of ˃7.8 mmol/L after 72 hr were considered to be diabetic rats for subsequent pharmacological testing, which were then randomized into three groups, including untreated diabetic (HFD/STZ, n=10), curcumin-treated (100 mg/kg BW/day) diabetic [(HFD/STZ) +Cur, n=10] groups and quercetin-treated (30 mg/kg BW/day) diabetic [(HFD/STZ) +Q, n=10] 31. The diabetic groups treated with curcumin and quercetin received their respective treatments in 0.5% carboxymethylcellulose buffer solution via oral gavage. The control rats received only 1% carboxymethylcellulose buffer. The rats were subjected to their diet for four weeks. After this period, they were sacrificed under diethyl ether anesthesia. The heart blood sample was taken to obtain the serum needed for biochemical testing. Liver, heart, skeletal, and renal muscles were immediately discarded and cleaned with a cold saline solution, and were then placed at −80°C for RNA extraction.
Quantitative real-time PCR (qPCR) for gene expression analysis: TRIzol reagent (Yektatajhizazma, Tehran, Iran) was used for total RNA extraction according to the manufacturer’s protocol. 2 μg RNA was subjected to reverse transcriptase to synthesize complementary DNA (cDNA). The process was based on the Yektatajhizazma protocol (Tehran, Iran), using stem-looped reverse transcription primers specific for mature microRNAs [Bonyakhteh, Tehran, Iran (Stem Cell Technology Research Center)]. Real-time PCR exploiting the master mix Super SYBR Green qPCR (Yektatajhizazma, Tehran, Iran) followed the thermal cycling profile: 95°C for 20 s, and then 40-cycle amplification (95°C for 10 s, 60°C for 10 s, and 72°C for 20 s). The SYBR Green fluorescence was tested for absorption in each tube at the end of each cycle. The β-actin gene, as a housekeeping gene, was selected to be an internal control to normalize the G6PD gene, and U87 was used to normalize examined miRNAs. The relative quantity of gene expression was obtained according to the method of 2−ΔΔCT. Forward and reverse primer sequences of U87, miR-1, miR-206, and miR-122 belonged to Bonyakhteh (Tehran, Iran (Stem Cell Technology Research Center)). The reverse primers of miR-1, miR-122, and miR-206 were a monopoly on the Company (Table 1).
Statistical analysis: One-way analysis of variance (ANOVA) was used to explore the differences between the groups (mean± SEM), followed by Tukey-Kramer multiple comparisons in GraphPad Prism9 software. Furthermore, the correlation of G6PD with miR-1, miR-122, and miR-206 was determined using SPSS software. p˂0.05 was considered to be a statistical significance level.
Results :
Curcumin and quercetin increased G6PD mRNA expression in liver, renal, heart, and muscle tissues of diabetic rats: Real-time PCR was employed to determine the mRNA expression level of G6PD. Following the β-Actin normalization, data revealed a significant decrease in the G6PD expression level in HFD/STZ groups compared with the controls (p=0.05). In the diabetic rats, quercetin treatment led to a notable upregulation of G6PD expression compared with HFD/STZ groups. Similarly, curcumin treatment significantly increased G6PD expression in heart and muscle tissues compared to the HFD/STZ groups. While analysis of the renal G6PD expression in curcumin-treated rats demonstrated an ascendant shift, the increase, however, did not reach statistical significance (Figure 1).
Curcumin and quercetin decrease miR-1, miR-122, and miR-206 expression in the liver, renal, heart, and muscle tissues of diabetic rats: Real-time PCR was recruited to determine miR-1, miR-122, and miR-206 mRNA expression levels, a reference gene of U87 normalized results. A comparison of miRNA expression levels between control and HFD/STZ groups revealed that miR-1, miR-122, and miR-206 expression levels were significantly elevated (except in renal tissue) (p<0.05). Following drug treatment, miR-1, miR-122, and miR-206 significantly decreased in the HFD/STZ+Cur and HFD/STZ+Q (p<0.05) compared with the HFD/STZ group. However, in the quercetin-treated group, a decline in miR-206 expression was observed but was not statistically significant in the heart tissue. Curcumin and quercetin had nearly identical impacts in changing the miR-1, miR-122, and miR-206 expression patterns in the HFD/STZ+Cur and HFD/STZ+Q groups, and no significant difference existed between them (Figure 2).
Correlation of G6PD with miR-1, miR-206, and miR-122: By analyzing the impact of intervention between the groups, SPSS software was used to assess the correlation, the data of which revealed a significant inverse correlation of G6PD with miR-122 in skeletal muscle, heart, and liver; with miR-206 in skeletal muscle and heart; and with miR-1 in skeletal muscle (Figure 3).
Discussion :
In response to the escalating prevalence and ongoing challenges in managing T2DM, the aim of the present work is to investigate curative and diagnostic approaches. Accordingly, the impacts of curcumin and quercetin on miR-1, miR-206, miR-122, and G6PD expression was assessed in the Control, HFD/STZ, HFD/STZ+treatment with curcumin supplementation groups, HFD/STZ+treatment with quercetin supplementation groups in the liver, renal, heart, and muscle in Wistar rats with T2DM.
G6PD, through its substantial NADPH generation, plays a vital role in supporting numerous NADPH-dependent cellular processes. As the primary cellular reductant, the adequate production of NADPH is crucial for maintaining the entire antioxidative system 32. There is considerable evidence suggesting that T2DM and G6PD deficiency augment each other. However, the precise underlying mechanism remains incompletely elucidated 33. An increase in cAMP in response to high glucose initiates the activation of PKA. Phosphorylation and subsequent inhibition of G6PD by protein kinase diminish intracellular NADPH levels, elevate ROS levels and oxidative stress, which ultimately leads to cell damage and death 2,34. The present study data revealed that G6PD expression was significantly downregulated in the HFD/STZ group compared with the control group in all tissues. Emerging preclinical and clinical data suggest that curcumin and quercetin possess the potential to mitigate diverse diabetic complications by lowering the levels of blood glucose through several mechanisms 35. Moreover, by exhibiting anti-apoptotic, anti-inflammatory, antioxidant, and anti-tumor properties, these compounds have substantial potential for therapeutic application in diabetes management 36-38. Many of these existing studies have focused on cancer cell lines, and research on the efficacy of curcumin and quercetin, specifically regarding
their ability to enhance G6PD expression for improving diabetes therapy, remains inadequate 39. Therefore, increased efforts in animal studies and comprehensive clinical trials should be directed toward this area. Here, the primary concept focuses on the potential of these compounds as anti-diabetic therapies, specifically targeting and modulating G6PD expression. As part of this research, administration of curcumin and quercetin treatment resulted in a significant upregulation of G6PD expression compared with control groups, observed across nearly all the tissues examined. Sark Tamova et al observed that quercetin elevates G6PD expression through Nrf2 signaling in human umbilical vein endothelial cells 40. An examination of DNA microarrays on C6 rat glioma cells revealed that curcumin upregulates G6PD expression 41. Yildirim et al detected higher levels of G6PD in Dextran Sodium Sulfate (DSS)-induced colitis mice fed with curcumin supplement compared to the control groups; however, the difference was not remarkable 42.
MicroRNAs have the potential to function as modulators of gene expression 43. Dysregulated miRNAs may be intricately linked to metabolic dysfunctions such as T2DM. MiR-1 and miR-206 play a regulatory role in glucose metabolism and insulin resistance, two critical factors in the pathogenesis of diabetes. miR-1 has emerged as a potential biomarker for pre-diabetes due to its positive correlation with blood glucose parameters and insulin resistance 44. miR-206 has been demonstrated to specifically regulate the activity of glucokinase, a critical enzyme involved in glucose-stimulated insulin secretion 45. In this study, there was a marked elevation in miR-1 and miR-206 in HFD/STZ groups compared with the control groups, supporting existing evidence that elevated glucose levels could upregulate miR-1 and miR-206 via SRF and MEK1/2 pathways 21. This finding supports previous studies, which demonstrated elevated circulating miR-1 levels in pre-diabetic individuals with impaired fasting glucose and glucose tolerance, and in the lipid-loaded myocardium of HFD mice 44,46. Furthermore, the analysis of miR-206 expression revealed a significant upregulation in the skeletal muscle tissue of patients diagnosed with T2DM 47. However, a previous study identified that expression levels of miR-1 were decreased in cardiomyocytes of diabetic rats 48. This may be attributed to myocardial damages, which dispatch miR-1 into the serum of patients 49.
Several recent investigations have also shown the effective role of quercetin and curcumin on various miRNA expressions across various disorders 50,51. In the present study, significant decrease in miR-1 and miR-206 was observed in almost all of the treated groups compared with HFD/STZ groups, which points towards the positive impact of the drugs.
Research has shown that overexpression of miR-206 and miR-1 inhibits cell proliferation by directly targeting G6PD, functioning as a tumor suppressor in cervical cancer 52,53. Previous studies have reported that miR-1 binds the 3′-UTR of G6PD, resulting in the suppression of G6PD expression by inhibiting its translation 17,54. Data from the present study showed a negative correlation of G6PD with miR-1 and miR-206. However, a significant inverse correlation of G6PD with miR-1 in skeletal muscle and miR-206 in heart and skeletal muscle was observed. This may be due to miR-1 and miR-206 being specific and highly expressed in the cardiac and skeletal muscles 18,20,55. Besides, existing literature reports relatively low baseline expression levels of miR-1 in the liver compared to other tissues 56.
Based on previous studies, miR-122, a liver-specific miRNA, targets G6PD and lowers its expression 23. A substantial rise in serum miR-122 has been observed in patients with diabetic retinopathy 57. Wang et al revealed that upregulated miR-122 exists in high glucose-triggered ARPE-19 cells 58. In support of these studies, present study results suggested that miR-122 is overexpressed in HFD/STZ groups in all tissues. The treated groups showed a significant decrease in miR-122 expression compared with HFD/STZ groups as previously proposed by other authors 59. Also, a significant inverse correlation of G6PD with miR-122 was found in all tissues except renal, suggesting a regulatory role of miR-122 through downregulating G6PD expression in diabetes. This assertion reflects results from other studies that suggested G6PD as a functional target of miR-122 23. However, the molecular mechanism of complex networks of miRNAs that regulate G6PD gene expression or other miRNAs is still a significant challenge that needs to be understood and requires further investigation.
Conclusion :
This study indicates a potential effect of curcumin and quercetin on controlling hyperglycemia by increasing G6PD expression and decreasing miR-1, miR-206, and miR-122 expression, which may lower oxidative stress. Also, findings showed an inverse correlation between miR-1, miR-206, and miR-122 levels and G6PD expression, suggesting a potential role for the miRNAs-G6PD network in anti-diabetic therapy and alleviating associated complications. However, further investigations might be necessary to better explain the association of G6PD with miR-1, miR-206, and miR-122 expression and the effect of curcumin and quercetin on this pathway.
Acknowledgement :
The authors would like to express their gratitude to Shahid Sadoughi University of Medical Sciences, Yazd, Iran, for providing research facilities and financial support for the first author’s MSc thesis. Approval was granted by the Research Ethics Committees of the Vice-Chancellor for Research Affairs - the International Campus of Shahid Sadoughi University of Medical Sciences in Yazd, Iran [IR.SSU.MEDICINE.REC. 1400.151].
Funding: This work was supported by the International Campus of Shahid Sadoughi University of Medical Sciences in Yazd, Iran (Grant numbers: 11014).
Conflict of Interest :
The authors declare no conflict of interest.
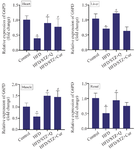
Figure 1. Gene expression of G6PD in heart, liver, skeletal muscle, and renal tissues in four studied groups (n=10) (Control, HFD, HFD/STZ+Q, HFD/STZ+Cur). Data are expressed as mean±SD.
* p-value <0.05 compared with the control group, # p-value <0.05 compared with the HFD group. HFD: High fat diet, STZ: Streptozotocin, Cur: Curcumin.
|
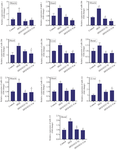
Figure 2. Gene expression of miR-1, miR-206, and miR-122 in heart, liver, skeletal muscle, and renal tissues in four studied groups (n=10) (Control, HFD, HFD/STZ+Q, HFD/STZ+Cur). Data are expressed as mean±SD.
* p-value <0.05 compared with the control group, #p-value <0.05 compared with the HFD group. HFD: High fat diet, STZ: Streptozotocin, Cur: Curcumin.
|

Figure 3. Association of miR-1, miR-206, and miR-122 with G6PD expression in four tissues (heart, muscle, liver, and renal). p<0.05 is considered significant.
|
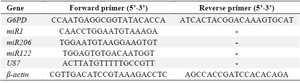
Table 1. Sequences of primers in real-time PCR
|
|