Synthesis of a Unique Dextran Polymer-Conjugated Antibody and Horseradish Peroxidase Complex
-
Zamani, Bahareh
-
Monoclonal Antibody Research Center, Avicenna Research Institute, Academic Center for Education, Culture and Research (ACECR), Tehran, Iran
-
Agharezaee, Niloofar
-
Monoclonal Antibody Research Center, Avicenna Research Institute, Academic Center for Education, Culture and Research (ACECR), Tehran, Iran
-
Moosavi, Farshid
-
Monoclonal Antibody Research Center, Avicenna Research Institute, Academic Center for Education, Culture and Research (ACECR), Tehran, Iran
-
Zamani Koukhaloo, Saeideh
-
Monoclonal Antibody Research Center, Avicenna Research Institute, Academic Center for Education, Culture and Research (ACECR), Tehran, Iran
-
Yousefi, Parisa
-
Monoclonal Antibody Research Center, Avicenna Research Institute, Academic Center for Education, Culture and Research (ACECR), Tehran, Iran
-
 Mahmoudian, Jafar
Monoclonal Antibody Research Center, Avicenna Research Institute, Academic Center for Education, Culture and Research (ACECR), Tehran, Iran, Tel: +98 21 22432020, Fax: +98 21 22432021; E-mail: j.mahmoudian@avicenna.ac.ir, jafarmahmoudian@gmail.com
Mahmoudian, Jafar
Monoclonal Antibody Research Center, Avicenna Research Institute, Academic Center for Education, Culture and Research (ACECR), Tehran, Iran, Tel: +98 21 22432020, Fax: +98 21 22432021; E-mail: j.mahmoudian@avicenna.ac.ir, jafarmahmoudian@gmail.com
Abstract: Background: Immunohistochemistry (IHC) is a practical technique that utilizes the specific binding between an antigen and antibody to detect and localize specific antigens in tissue and cells. The optimal sensitivity in IHC is of utmost importance to achieve reliable results even when antigens are present at low abundance on the samples. Here, a dextran polymer labeled with Horseradish Peroxidase (HRP) and an anti-body to improve the sensitivity of the IHC technique was synthesized.
Methods: To this end, free thiol groups were introduced on sodium periodate-activated 30 kDa dextran using cystamine, followed by attachment of sulfo-MBS-activated goat anti-mouse antibody and sulfo-MBS-activated HRP to the activated dextran.
Results: The production of Poly-HRP Antibody (PHA) was confirmed by the appearance of a new protein band exceeding 150 kDa on Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE). Additionally, Enzyme-Linked Immunosorbent Assay (ELISA) and IHC techniques were employed to characterize PHA’s functionality. The data demonstrated that PHA effectively detected target antigens in these assays.
Conclusion: The newly synthesized PHA has the potential to provide a more sensitive platform for early detection of biomarkers in IHC. Further research is needed to evaluate its cost-effectiveness.
Introduction :
During the past few decades, many investigators have attempted to increase the sensitivity of detection systems in the Immunohistochemistry (IHC) technique 1-3. IHC is a powerful diagnostic tool for detecting different antigens like cancer biomarkers. This technique is mostly applied in early cancer detection with applications spanning clinical diagnosis, research, and dis-ease monitoring 4,5. The specific activity of enzyme-antibody conjugates directly influences the sensitivity of the IHC technique. Notably, in conventional Horse-radish Peroxidase (HRP)-antibody conjugates, only three HRP molecules may be conjugated to one IgG molecule using periodate, benzo-quinone, or thiol-maleimide approaches because of the steric hindrance of the antibody 6,7. This steric hindrance not only restricts the number of enzymes conjugated but also in-fluences the availability of functional groups such as primary amines, sulfhydryls, sugars, and polysaccharides required for conjugation 8-10. While this molar ratio is adequate for techniques like ELISA, achieving the high sensitivity needed for detecting targets in the low picogram to femtogram range necessitates the development of novel conjugation strategies that can overcome these steric limitations 11,12.
To date, different detection systems have been employed to increase the sensitivity and signal amplification in the IHC technique including Peroxidase Anti-Peroxidase complex (PAP), Labeled Avidin-Biotin (LAB), Labeled Streptavidin-Biotin (LSAB), and Avidin-Biotin Complex (ABC) methods 13. Due to the use of peroxidase enzyme in the PAP method, false positive results might be observed 14. As a result, PAP might not be suitable to tissues with high endogenous peroxidase such as bone marrow and spleen 15. Regarding LAB and LSAB, tissues and cells with high amounts of biotin, such as the placenta and mammary glands are susceptible to attaching to avidin/streptavidin, which results in the nonspecific staining 15-17. In these assays, using a biotin-blocking reagent is highly recommended to prevent background 18-21. Unlike LAB and LSAB in which only one conjugate is used in the detection procedure, a lattice complex in ABC is con-structed to amplify the signal 22-24. Recently, an HRP-polymer conjugate has emerged as a powerful approach for signal amplification in IHC. The HRP-polymer conjugate can attach approximately 40 moles of HRP and 11 moles of antibody molecules to one mole of Dextran polymer 25. Conjugation of many HRPs and antibodies to a single polymer might improve the sensitivity, efficiency, and reliability of the assay 26. In the current study, a novel approach to synthesize a dextran polymer-based detection system with enhanced sensitivity for IHC applications was presented. The strategy involves the development of a poly-HRP antibody conjugate through a controlled chemical process, with subsequent validation using SDS-PAGE, ELISA, and IHC. The method is summarized in a graphical abstract (Figure 1).
Materials and Methods :
The production of goat anti-mouse IgG: Production, purification, and characterization of a polyclonal antibody were achieved according to our previous experiment 27. Briefly, a goat was immunized with a protein G-purified mouse IgG. Freund's complete and incomplete adjuvants (Sigma-Aldrich, Burlington, MA, United States) were used to immunize the goat according to the manufacturer's instructions. To purify the goat anti-mouse IgG polyclonal antibody from goat serum, a mouse IgG-coupled Cyanogen bromide (CNBr)-activated Sepharose 4B column was prepared according to the manufacturer protocol (GE Healthcare, Chicago, Illinois, United States). Notably, to eliminate the non-specific binding of the goat antimouse antibody, the purified goat anti-mouse IgG was adsorbed using a human IgG column. Following the adsorption of the goat anti-mouse IgG, the presence and reactivity of the purified- and adsorbed- antibodies were assessed using SDS-PAGE and ELISA techniques, respectively. For ELISA, mouse IgG, 1 μg/ml, was coated and then blocked with 5% Bovine Serum Albumin (BSA). The purified goat anti-mouse IgG antibody was diluted in a range of 1-0.03 μg/ml. Finally, for detection, an HRP-labeled rabbit anti-goat IgG (DAKO, Glostrup, Denmark), at a dilution of 1/5000, was used and TMB (3, 3’, 5, 5’-Tetramethylbenzidine) was applied as an enzyme substrate.
The production of poly HRP-Antibody (PHA) conjugate: Sixty mg of dextran (Sigma-Aldrich), with a molecular weight of 30 kDa, was dissolved in water (20 mg/ml) and then oxidized with 60 mM sodium periodate at Room Temperature (RT) overnight in the dark. The solution was dialyzed against water for two days at RT. The concentration of the oxidized dextran was then determined using the phenol-sulfuric acid method. For this purpose, the activated dextran was diluted 1:500 in distilled water, and then 50 μl of Phenol and 5 ml of sulfuric acid were added. The Optical Density (OD) of the solution was measured at 490 nm. Cystamine was used to produce free thiol groups on the oxidized dextran molecules a pH of 7.4-7.6. To achieve this, Cystamine was first reduced by 50 mM sodium cyanoborohydride to generate two distinct free thiol groups and then one mole of dextran was reacted with 100 moles of cystamine for one hr at RT. After three hours of shaking, the Schiff-base amination was reduced by 50 mM sodium cyanoborohydride for one hour. Subsequently, the thiolated dextran was dialyzed against water supplemented with 10 mM EDTA overnight at RT. To measure the production of free thiol groups, the Ellman assay was performed according to the standard protocol 28. HRP (Sigma-Aldrich) and the human IgG-adsorbed goat anti-mouse IgG were activated by m-maleimidobenzoyl-N-hydroxysulfosuccinimide ester (sulfo-MBS linker). Briefly, 200 μg of sulfo-MBS was dissolved in water and then added to a mixture of 1 mg antibody, 10 mg/ml, and 1.2 mg HRP, 10 mg/ml, in Phosphate-buffered saline (PBS) for two hr with shaking at RT. The mixture of linker-activated HRP and antibody was dialyzed against PBS containing 10 mM EDTA overnight at 4°C. The final concentration of HRP-MBS and antibody-MBS were then measured at 280 nm using a spectrophotometer (Eppendorf, Bio-photometer, Hamburg, Germany). Finally, to produce a poly HRP-antibody, one mole of the thiolated dextran, 80 moles of HRP-MBS, and 20 moles of MBS-activated goat anti-mouse antibody were mixed for six hr at 4°C.
The characterization of PHA by SDS-PAGE: A mixture of MBS-activated HRP (10 μg) and MBS-activated goat anti-mouse IgG (10 μg) as well as PHA (10 μg) were separately mixed in a sample buffer containing 150 mM Tris (pH=6.8), 1.2% SDS, 33% glycerol, and 0.2% bromophenol blue and then boiled for 10 min. Samples (20 μl) were run on a 10% SDS-PAGE gel at 100 V for one hr. The gel was stained with Coomassie Blue for one hr with gentle agitation and then destained in a destaining solution until the protein bands became visible.
The functional assessment of PHA by ELISA: The reactivity of PHA against mouse IgG was evaluated by a direct ELISA. A Plate (Nunc, Roskilde, Denmark) was coated with mouse IgG, 5 μg/ml, and then incubated at 4°C overnight. After washing three times with PBS containing by 0.1% Tween 20, the wells were blocked with 5% BSA at 37°C for two hr. The PHA was diluted in a range of 5-0.15 μg/ml and incubated for one hour at 37°C. For the negative control, mouse IgG antibody was omitted. The wells were washed and then Tetramethylbenzidine (TMB) was added to each well. The reaction was stopped adding 20% H2SO4. The Optical Density (OD) was measured at 450 nm using an ELISA reader (BioTek, Winooski, VT).
The assessment of the PHA efficiency by IHC: IHC was carried out to assess the efficiency of PHA on paraffin-embedded slides of MDA-MB-231 tumor using the chick embryo Chorioallantoic Membrane (CAM). The slides were first deparaffinized using Xylene for 5 min and then rehydrated by soaking in ethanol solutions (from 100 to 50%) step-by-step, ending with Double Distilled Water (DDW). The antigen retrieval was accomplished through a heat-induced method by incubating the slides in 10 mM citrate buffer (pH=6), at 95°C, for 30 min. Following washing with Tris-buffered saline (TBS) buffer, all slides were incubated with 3% H2O2 in the dark for 15 min to block endogenous peroxidase activity. Prior to the primary antibody incubation, the slides were blocked using the background blocker (DAKO) for 10 min with blocking solution. After washing, the anti-vimentin monoclonal antibody (ARI, Tehran, Iran), 10 μg/ml, was incubated at RT, for 45 min. For signal amplification, a mouse/ rabbit enhancer solution (Dako) was added to the slides for 10 min. After washing, the PHA (200 μg/ml) were added for 45 min at RT. Finally, the DAB (diaminobenzidine) chromogen was applied for 10 min. For the negative controls, anti-vimentin monoclonal antibodies were omitted. The images were visualized using a light microscope (Olympus, Tokyo, Japan).
Results :
Production of the goat anti-mouse IgG antibodies: The goat anti-mouse IgG antibody, 150 kDa, was purified from hyper-immunized goat serum (Figure 2A). Because the goat anti-mouse IgG antibodies cross-react with human IgG, the purified goat anti-mouse IgG antibody was adsorbed with human IgG to eliminate their non-specific binding (essential for IHC on human tissues) (Figure 2B). Subsequent SDS-PAGE revealed that no other extra protein bands were co-purified with the antibody (Figure 2C). The reactivity of both the non-adsorbed and human IgG-adsorbed goat anti-mouse IgG antibodies was assessed by ELI-SA (Figure 2D). ELISA results showed no difference in reactivity between the adsorbed and non-adsorbed antibodies.
Characterization of PHA by SDS-PAGE and ELISA: The conjugate efficacy and functional reactivity of PHA were assessed by SDS-PAGE and ELISA, respectively. Based on SDS-PAGE result, figure 3A, the appearance of new protein bands (>150 kDa) above intact antibody, human IgG-adsorbed goat anti-mouse IgG antibody, represented the conjugation of HRP and anti-body to the dextran polymer. In addition, the reactivity of PHA was investigated by ELISA (Figure 3B). The ELISA results clearly showed that the PHA was able to recognize the antigen, mouse IgG, properly.
Efficiency confirmation of PHA by IHC assay: The functionality of the PHA was evaluated using IHC, as illustrated in figure 4. In this study, vimentin antigen was targeted with a monoclonal anti-vimentin antibody applied to MDA-MB-231 tumor cells on the chick embryo Chorioallantoic Membrane (CAM). The human IgG-adsorbed anti-mouse IgG antibody of the PHA was reacted with the monoclonal anti-vimentin antibody. In the current test, in the negative control (Figure 4A) anti-vimentin monoclonal antibody was excluded, whereas in figure 4B, the anti-vimentin monoclonal antibody was included to assess the PHA's functionality in IHC. Notably, a commercial conjugate was used in both negative (Figure 4C) and positive (Figure 4D) controls to compare with the PHA results.
Discussion :
IHC is the most common application of immunostaining. In IHC, antigens (proteins) are selectively identified in cells of tissue sections 5. However, this method has some limitations including low availability of antigens on the sample, antigen masking during fixation and embedding, and low antigen-antibody binding 22. Among different restrictions in the IHC method, one of the most important limitations is its low sensitivity because of limited signal production. To overcome this limitation, polymer-based conjugates have emerged for signal amplification.
One of the common complications in the IHC technique is a non-specific background resulting from the non-specific binding of conjugates 13. In this study, to avoid the non-specific interaction of the goat anti-mouse IgG with human IgG molecules on human tissue slides, the goat anti-mouse IgG was adsorbed by hu-man IgG with a human IgG column. Thus, the adsorbed antibodies enabled the PHA to perform specifically in the IHC.
Heterobifunctional crosslinkers, like MBS, are used to couple molecules through amine and sulfhydryl groups. This enables specific sequential reactions of two molecules without polymerization and self-interactions 29. MBS contains N-hydroxysuccinimide (NHS) and maleimide groups, which react with primary amines and sulfhydryl groups, respectively 29. The advantage of MBS is that it allows selective and stable linking of antibodies and HRPs via surface amines, while the thiol-functionalized dextran binds to the protein-attached maleimide groups. Considering these factors in our design, any possible self-interaction between antibody and HRP molecules was prevented.
Notably, the conjugation of a distinct number of antibodies (150 kDa) and HRPs (45 kDa) to the dextran polymer (40 kDa) resulted in the appearance of complex molecules with high molecular weights. In our study, SDS-PAGE (Figure 3A) demonstrated the appearance of many protein bands above 150 kDa representing antibody-HRP-dextran complexes. This finding is in line with other investigations representing the protein bands greater than 150 kDa in SDS-PAGE 30; In our SDS-PAGE (Figure 3A), the 150 and 45 kDa protein bands indicate the un-conjugated antibodies and HRPs, respectively.
In 2012, Fahimeh Charbgo et al developed a poly HRP-streptavidin conjugate to use in ELISA and IHC 30. Although the attachment of streptavidin to the poly- HRP complexes could provide the capability to apply different antibodies in immunoassays, the main restriction is that antibodies must be biotinylated in order to be used in these tests. In addition, the streptavidin might react with endogenous biotin in tissues and result in falsely overestimated signals 30. To overcome the aforementioned problem, we conjugated the human IgG-adsorbed goat anti-mouse antibodies to poly-HRP complex in order to recognize mouse monoclonal antibodies in immunoassay experiments directly. Notably, nowadays most antibodies that interact with their respective antigens on tissue samples, are produced in mice using hybridoma technology. As a result, our conjugate could be applied to IHC methods without any further experiments, making it more convenient.
Conclusion :
In the current study, an efficient and easy-to-use conjugation approach was developed to enhance sensitivity in immunostaining-based detection systems such as IHC. This aim was achieved through the thiolation of the dextran polymer by cystamine and the conjugation of activated-HRP and-antibody molecules to the SH-functionalized dextran backbone. Finally, the poly- HRP antibody conjugate (PHA) was characterized and confirmed by SDS-PAGE, ELISA, and IHC. We believe that our newly synthesized PHA could be a valuable tool for researchers working in protein/antigen detection with immunostaining techniques. Moreover, this method holds promise for broader applications in diagnostic pathology and research, potentially enabling more sensitive detection of low-abundance biomarkers and facilitating monitoring and earlier diagnosis of diseases.
Ethical approval :
All procedures conducted in this research were approved by ethical committees of Avicenna Research Institute (IR.ACECR.AVICENNA.REC.1398.030).
Acknowledgement :
This research has been funded by Avicenna Re-search Institute (ARI). We send many appreciations to our colleagues at ARI for their support. The authors declare no conflict of interests.
Conflict of Interest :
The authors declared no conflict of interest.
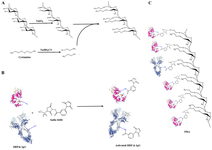
Figure 1. Shematic illustration of the PHA production. Dextran polymer was oxidized by sodium peridate and then thiolated using cystamine by schiff-base amination (A). Antibodies and HRPs were activated using sulfo-MBS linker (B). Final PHA structure (C).
|
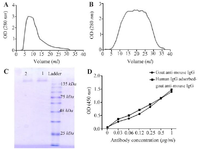
Figure 2. Antibody purification and characterization. Goat anti-mouse IgG was purified from the hyper-immunized goat serum using mouse IgG column (A) and then the goat anti-mouse IgG was cross-adsorbed against human IgG using human IgG column (B). The purity and the reactivity of both goat anti-mouse IgG (C, lane 1 in SDS-PAGE) and human IgG-adsorbed goat anti-mouse IgG (C, lane 2 in SDS-PAGE) antibodies were examined using SDS-PAGE (C) and ELISA, (D) respectively.
|
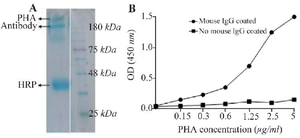
Figure 3. PHA characterization using SDS-PAGE and ELISA. SDS-PAGE analysis showed the presence of a protein bands above the intact antibody, 150 kDa, indicating the conjugation of antibody and HRP to dextran polymer (A). Using ELISA revealed that the PHA could recognized the corresponding antigen, mouse IgG (B).
|
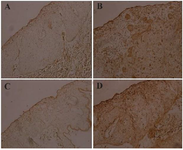
Figure 4. Evaluation of PHA functionality using IHC. The targeting of vimentin antigen with monoclonal anti-vimentin antibody on MDA-MB-231 tumor cells on chick embryo CAM was examined. Negative control without anti-vimentin antibody (A). Test sample including anti-vimentin antibody to assess PHA activity (B). Negative control using a commercial conjugate (C). Positive control with a commercial conjugate (D).
|
|