Characterization and Evaluation of the Anti-proliferative Activity and Hypersensitivity of L-Asparaginase from Trichosporon asahii Isolate ChL11 and Candida palmioleophila Isolate JK12
-
 Sisay , Tekeba
Department of Molecular Biology & Biotechnology, Pan African University Institute for Basic Sciences, Technology and Innovations (PAUSTI), Nairobi, Kenya, Tel: +25 1918276473; E-mail: 21sisay@gmail.com, tekeba.sisay@uog.edu.et
Sisay , Tekeba
Department of Molecular Biology & Biotechnology, Pan African University Institute for Basic Sciences, Technology and Innovations (PAUSTI), Nairobi, Kenya, Tel: +25 1918276473; E-mail: 21sisay@gmail.com, tekeba.sisay@uog.edu.et
-
Department of Medical Biotechnology, Institute of Biotechnology, University of Gondar, Gondar, Ethiopia
-
Maina, Naomi
-
Department of Molecular Biology and Biotechnology, Pan African University Institute for Basic Sciences, Technology and Innovations (PAUSTI), Nairobi, Kenya
-
Biochemistry Department, College of Health Sciences, Jomo Kenyatta University of Agriculture and Technology, , Nairobi, Kenya
-
Mobegi, Victor
-
Department of Biochemistry, Faculty of Science and Technology, University of Nairobi, Nairobi, Kenya
-
Wachira, Sabina
-
Kenya Medical Research Institute, Center for Traditional Medicine and Drug Research, Nairobi, Kenya
Abstract: Background: L-Asparaginase is a crucial enzyme to treat Acute Lymphoblastic Leukemia (ALL), as it depletes L-asparagine, an essential amino acid for cancer cell survival. However, its clinical use is often restricted due to hypersensitivity reactions. This study examined the anti-proliferative effects and hypersensitivity of fungal L-asparaginases (L-ASNases) from Trichosporon asahii isolate ChL11 (TaIChL11 L-ASNase) and Candida palmioleophila isolate JK12 (CpIJK12 L-ASNase).
Methods: The enzymes were produced and purified through ammonium sulfate precipitation, dialysis, and Sephadex G-100 chromatography, and tested on leukemia cells and BALB/c female mice to assess immune responses.
Results: TaIChL11 L-ASNase had a molecular weight of 40 kDa, Michaelis constant (KM) of 1.66×10⁻² mM, and Vmax of 37.23 mM/min, while CpIJK12 L-ASNase had a molecular weight of 135 kDa, KM of 2.3×10⁻² mM, and Vmax of 14.03 mM/min. Both enzymes exhibited significant anti-proliferative effects against CCRF-CEM cancer cells, with half-maximal inhibitory concentration (IC50) values of 2.74 U/ml for TaIChL11 L-ASNase and 3.30 U/ml for CpIJK12 L-ASNase after 48 hr, improving further after 72 hr. They also showed low cytotoxicity toward normal Vero E6 cells. in vivo studies demonstrated that TaIChL11 ASNase-treated mice had significantly lower Immunoglobulin (Ig) G levels than those treated with commercial L-ASNase from Erwinia chrysanthemi (Owenism) (p<0.005), with no detectable IgE response.
Conclusion: These findings indicate that fungal L-ASNases, particularly TaIChL11 ASNase, with lower L-glutaminase activity and a favorable safety profile, could be promising alternatives to bacterial L-ASNases, potentially enhancing ALL treatment with fewer side effects.
Introduction :
In biotechnology and pharmaceutical research, L-ASNase is a crucial enzyme capable of selectively hydrolyzing the amino acid L-asparagine into aspartic acid and ammonia 1. This enzymatic action is significant in cancer treatment, where rapid proliferation of malignant cells relies heavily on L-asparagine 2. By depleting this amino acid, L-ASNase effectively inhibits cancer cell growth, making it a valuable component of chemotherapy regimens 3.
Traditionally, L-ASNase has been sourced primarily from bacteria, notably Escherichia coli (E. coli) and Erwinia chrysanthemi 4. However, there are concerns over its immunological and hypersensitive reactions may be because of its prokaryotic nature and L-glutaminase (L-GLNase) activity 5,6. As a result of the side effects associated with bacterial-derived enzymes, there has been spurred interest in alternative sources, such as fungi 7. Numerous studies have shown that various fungal genera, such as Aspergillus, Penicillium, Cladosporium, Saccharomyces cerevisiae, Candida utilis (C. utilis), Trichosporon asahii (T. asahii), and Fusarium, possess L-ASNase 8-15. Furthermore, Freitas et al 16 identified fungal isolates, including Penicillium sizovae 2DSST1 and Fusarium (F) proliferatum DCFS10, which were reported to exhibit high L-ASNase activity along with low L-glutaminase (L-GLNase) activity. Doriya and Kumar 17 have also reported a fungal isolate designated as C7 that can produce L-ASNase free of urease or L-GLNase activity. Moreover, fungal isolates T. asahii isolate ChL11 and Candida palmioleophila (C. palmioleophila) isolate JK12 have been identified as promising L-ANSase producers with reduced L-GLNase activity 18.
Characterizing L-ASNase in terms of Michaelis constant (KM), maximum velocity (Vmax), substrate specificity, optimal reaction pH, and optimal reaction temperature is fundamental for optimizing its therapeutic application. These parameters influence the enzyme’s efficiency, specificity, and stability, which are key to its success in treating leukemia with minimal side effects 19. A lower KM value for L-ASNase signifies a higher affinity for the substrate, enabling the enzyme to achieve half of its maximum reaction rate at lower substrate concentrations 20. For therapeutic applications, particularly in treating Acute Lymphoblastic Leukemia (ALL), an ideal L-ASNase should have a millimolar KM value for L-asparagine, ensuring effective substrate affinity 21. Additionally, L-ASNase with high selectivity for L-asparagine and minimal L-glutamine activity is preferred, as this reduces immunogenicity and minimizes side effects associated with L-GLNase co-activity 1.
Temperature and pH are key factors influencing enzyme efficiency and effectiveness. pH can alter the enzyme's active site and substrate charge, affecting catalytic performance 22. Temperature also impacts enzyme kinetics, either boosting or reducing reaction rates depending on optimal conditions 23. Understanding these factors is crucial for optimizing L-ASNase activity in various applications. This study aimed to characterize L-ASNase produced by two fungal isolates, T. asahii isolate ChL11 and C. palmioleophila isolate JK12, previously isolated from soil samples18. Furthermore, this research focused on evaluating their anticancer potential, and hypersensitivity effects to assess their suitability for medical applications.
Materials and Methods :
Production and purification of L-asparaginase: The production of L-ASNase is influenced by various factors, including media components like carbon and nitrogen sources, as well as fermentation conditions such as incubation temperature, initial pH of the Modified Czapek Dox (MCD) media, fermentation duration, inoculum size, agitation speed, and the composition of the MCD media 24. This study used a One-Factor-at-a-Time (OFT) approach for optimization, with other factors held constant and incorporated into subsequent experiments. The optimization process was conducted as described by Sisay et al 18, and the production of L-ASNase was performed under the optimized conditions. The optimal conditions were assessed based on the enzyme activity produced using the standard method 25.
Following L-ASNase production under optimized conditions and media using the selected isolates, purification was conducted at 4°C, comprising several critical steps. Initially, the production broth was centrifuged to separate the supernatant from the cells and filtered with 0.22 µm pore size filter paper. Subsequently, ammonium sulfate was gradually added to the filtrate until it attained a saturation level of 60%. This mixture was then incubated overnight at 4°C before undergoing centrifugation at 11,000 rpm for 20 min 26. The resulting precipitate was dissolved in the minimum volume of 50 mM Tris-HCl buffer (pH=8.6) and underwent dialysis against the same buffer for 48 hr, with periodic replacement of the dialysis buffer (50 mM Tris-HCl buffer, pH=8.6) every 12 hr at 4°C using a 50 kDa cutoff to remove salts. After dialysis, the fraction was applied onto a Sephadex G-100 column pre-equilibrated with 0.05 M Tris-HCl buffer. Protein elution was achieved using 50 mM Tris-HCl buffer containing 0.1 M KCl. Afterward, fractions were collected and assessed for protein content and enzyme activity. The fraction demonstrating the highest enzyme activity was then subjected to lyophilization, resulting in a powder stored at 4°C 27.
Estimation of the total protein content and enzyme activity: The protein content of the enzyme was estimated by the Bradford method, as described by Bradford 28. Standard Bovine Serum Albumin (BSA) solutions (10 to 100 μg in 0.1 ml) were prepared and dispensed into test tubes. The volume was adjusted to 0.1 ml with phosphate buffer, and then 5 ml of protein reagent was added. Absorbance at 595 nm was measured after 10 min, using a blank of phosphate buffer and protein reagent 28. Protein concentration was determined by comparing it to a BSA standard curve.
The enzymatic activity of purified L-ASNase was assessed according to the Nesslerization method 25. The Nesslerization method entails preparing a reaction mixture in a test tube, consisting of 0.5 ml of the enzyme, 0.5 ml of 0.5 M Tris buffer, 0.5 ml of 0.04 M L-asparagine, and 0.5 ml of distilled water. This mixture was incubated for 30 min, and the reaction was stopped by adding 0.5 ml of 1.5 M Trichloroacetic Acid (TCA). After centrifugation at 12,000 revolutions/min (rpm) for 15 min, 0.1 ml of the supernatant was mixed with 3.7 ml of distilled water. Then, 0.2 ml of Nessler’s reagent was added, and the absorbance was measured at 436 nm using a spectrophotometer 29.
A standard curve was created using ammonium sulfate solutions (20-100 nM) to quantify liberated ammonia. For the control group, the reaction mixture was prepared similarly except for adding the enzyme after adding 1.5 M TCA to the mixture. One international unit (U) of L-ASNase activity is defined as the amount of enzyme needed to produce 1 µmol of ammonia per minute under the assay conditions. Enzyme activity was calculated using the formula described and the enzyme-specific activity was calculated by dividing the amount of enzyme (units) by the total protein concentration (mg).
Enzyme activity UmL=µmole of ammonia liberated2.200.2300.1
Where, 2.20= Final volume of the reaction system, 0.2 = volume taken for OD, 0.1= Enzyme aliquots, and 30 = Incubation time (in min).
Characterization of L-asparaginase: Determination of L-asparaginase molecular weight: The molecular weight of the purified enzyme was assessed through SDS-PAGE, relative to standard molecular markers. The L-ASNase samples were loaded onto the gel and separated at 70 V for 120 min, alongside a molecular mass marker ranging from 11 to 240 kDa. The enzyme's molecular mass was determined by comparing its migration distance with the molecular mass markers, as described by Laemmli 30.
Effect of temperature and pH on L-asparaginase reaction: The L-ASNase activity was measured at 20°C, 25°C, 30°C, 35°C, 40°C, and 45°C to evaluate the effect of temperature. The reaction conditions were maintained for 30 min, and the resulting ammonia was quantified employing Nessler’s reagent, a methodology by the protocol elucidated by Imada et al 25.
The pH profile of the enzyme was determined at 37oC in various pH (pH=5.0-10.0) using 0.5 mg ml−1 of enzyme solution (10 units/ml). The buffers used in this study were potassium phosphate (50 mM, pH=5-7.5) and Tri-HC1 (50 mM, pH=8.0-10). This evaluation involves 189 mM L-asparagine solution as a substrate 31. After 30 min of incubation, ammonia was quantified using the Nesslerization method by Imada et al 25.
Effect of substrate concentration on L-asparaginase activity: To investigate the effect of substrate concentration on enzyme activity, L-asparagine was tested at various concentrations: 1.25, 2.5, 5, 10, and 20 µM. Enzyme activity was measured using the Nesslerization method 23. Each assay was duplicated, and average values were used for kinetic analysis. The kinetic parameters, including the Vmax, the KM, and the enzymes' turnover number (Kcat) were determined using Lineweaver-Burk plots based on the Michaelis-Menten equation 32.
V=KM+[S]Vmax [S]
Kcat=VmaxEc
where: V is the reaction rate (velocity); [S] is the substrate concentration; Vmax is the maximum reaction rate; KM is the Michaelis constant; EC: Enzyme concentration; and Kcat: turnover number of enzymes.
Effect of incubation time on L-asparaginase activity: The impact of incubation time on the enzyme's activity was investigated by varying the duration of incubation while maintaining constant other factors. Incubation periods of 5, 10, 15, 20, 25, and 30 min were tested to assess the enzyme's performance over a range of time intervals. The reaction was carried out in 50 mM Tris Buffer, pH=8.6 at 37°C. When the incubation time reached, the reaction was stopped by 1.5 M Trichloroacetic Acid (TCA) solution and the enzyme activity was measured following standard methods 25,31.
Evaluation of substrate specificity of L-asparaginase: The determination of L-ASNase specificity for L-asparagine and L-glutamine was conducted to elucidate the enzyme's substrate preferences. In this experiment, the L-ASNase enzyme was incubated separately with 98 mM L-asparagine and L-glutamine in 0.05 M Tris-HCl buffer (pH=8.6) at 37°C for 30 min. The reactions were quenched, and the results were assessed using Nessler's reagent. The absorbance of the reaction mixtures was measured at 436 nm wavelengths 33.
Antiproliferative effect and hypersensitivity reaction of L-asparaginase: Cell culture media preparation and reviving cells: The RPMI-1640 media for the leukemia cell line was prepared according to the manufacturer's instructions by dissolving RPMI-1640 with 2 mM L-glutamine, 10 mM Hepes, 1 mM sodium pyruvate, 2.5 g/L glucose in sterile double-distilled water, and supplementing with 1% penicillin/streptomycin, 10% heat-inactivated Fetal Bovine Serum (FBS), and 2 g/L Sodium Bicarbonate 34. For the Vero E6 cell line, Dulbecco’s Modified Eagle’s Media (DMEM) was prepared by dissolving DMEM with 4.5 g/L glucose, 10% FBS, 1.5 mM L-glutamine, 10 mM Hepes, 2 g/L Sodium Bicarbonate, and 1% penicillin/streptomycin. After mixing, the pH of both RPMI and DMEM media was adjusted to 7.2-7.5 using 1 N HCl and then filtered 35.
The leukemia (CCRF-CEM) and non-cancerous (Vero-E6) cell lines were obtained from the Kenya Medical Research Institute (KEMRI). The growth media (RPMI-1640 for CCRF-CEM and DMEM for Vero-E6 cells) were warmed to 37°C for 30 min. Five ml of each pre-warmed medium were added to T75 culture flasks. The frozen cells were thawed in a 37°C water bath, disinfected with 70% ethanol, and then transferred to the T75 flasks. A 0.2 ml aliquot of each cell suspension was stained with trypan blue to count viable cells, and the cell density was adjusted to 1×104 cells/ml 36. The flasks were incubated at 37°C with 5% CO2, and cell growth was monitored daily using a phase-contrast microscope. Viable cells were counted at 0, 24, 48, and 72 hr to determine the exponential growth phase. The cells were then split into new flasks for further use.
Resazurin cell viability assay: The cytotoxicity and anticancer activities of purified L-ASNase were assessed on cancerous (CCRF-CEM) and non-cancerous (Vero-E6) cell lines using the resazurin assay 37. This assay measures cell viability by detecting the conversion of resazurin to resorufin by viable cells, resulting in a pink color change, which is quantified using fluorescence. Briefly, 1×104 cells in the exponential phase were seeded into 96-well plates with 100 μl RPMI-1640 for CCRF-CEM cells and DMEM for Vero-E6 cells, and incubated for 24 hr. The cells were then treated with various concentrations of L-ASNase (0.78-100 U/ml) in triplicates, with a control group included. The plates were incubated at 37°C with 5% CO2 for 24, 48, or 72 hr. After incubation, 20 μl of resazurin solution (1 mg/ml in PBS) was added to each well and incubated for an additional 5 hr. The absorbance was measured at 560 nm and 600 nm using an ELISA reader, and cell viability was calculated. The IC50 (half-maximal inhibitory concentration) for cancer cells and CC50 (half-maximal cytotoxic concentration) for normal cells were determined, and the Selectivity Index (SI) was calculated by dividing the CC50 of normal cells by the IC50 of cancer cells 38.
Hypersensitivity reactions to L-asparaginase: L-asparaginase sensitization: The sample size was calculated using the formula n=DF/K+1, where K is the number of groups and DF is the degrees of freedom 39. The study used twenty-four female BALB/c mice, aged 5-8 weeks and weighing 20-30 g, obtained from the JKUAT Small Animal Facility for Research and Innovations (SAFARI), which were randomly divided into four groups. In the study, Group 1 (negative control) received intraperitoneal injections of 100 μl Freund’s adjuvant mixed with 100 μl normal saline solution (0.9% NaCl) on days 1 and 14. Groups 2, 3, and 4 were sensitized with 56.25 U/kg L-ASNase on day 1 and 112.5 U/kg on day 14, both with 100 μl Freund’s adjuvant, using L-ASNase from T. asahii isolate ChL11, C. palmioleophila isolate JK12, or a commercial source. Then 100 µl blood samples were collected from the tail vein of all the groups on days 13 and 23 for further analysis.
Induction of antibody production and blood collection: After sensitization, Group 1 received a challenge of 100 μl normal saline solution. In contrast, Groups 2, 3, and 4 were challenged with 1125 U/kg (100 μl) L-ASNase (from T. asahii isolate ChL11, C. palmioleophila isolate JK12, or commercial source) on day 24. Then, 100 μl blood samples were collected via cardiac puncture on day 28. Serum was obtained by centrifugation at 400×g and 4°C for 10 min.
ELISA assessment of antibody production: The levels of IgG and IgE production against L-ASNase were quantified using an ELISA kit from Solarbio Life Science. Briefly, 100 μl of standard, blank, or diluted serum samples were added into wells of 96-well plates precoated with each specific antigen. After incubation for 1 hr, 100 μl of enzyme-antibody conjugate was added to each well. Subsequently, 100 μl of TMB (3,3′, 5,5′-Tetramethylbenzidine) substrate solution was added into each well, and incubated at room temperature for 10 E. coli. Finally, 100 μl of stop solution was added to each well, and the absorbance was measured at 450 nm using an ELISA Reader 40,41.
Statistical analysis: All experiments were conducted in replicates of each parameter, allowing for the calculation of standard deviation and standard error of the mean to provide insight into the variability within each independent variable. Enzyme kinetic parameters were calculated using the Lineweaver-Burk plot in R.
Results :
Optimization of media and culture conditions for L-asparaginase production: In this study, two fungal isolates, T. asahii isolate ChL11 and C. palmioleophila isolate JK12, were used to produce L-ASNase under optimized conditions, as shown in table 1. For T. asahii isolate ChL11, the optimal conditions were 35°C, 120 hr incubation, initial pH=8.0, 2.0% inoculum, and 50 rpm agitation, with sucrose and L-asparagine as the carbon and nitrogen sources, respectively. The optimal conditions for C. palmioleophila isolate JK12 were 25°C, 96 hr incubation, initial pH=6.5, 8.0% inoculum, and 200 rpm agitation, with fructose and yeast extract as the carbon and nitrogen sources.
Purification and characterization of L-asparaginase: The culture filtrate from T. asahii isolate ChL11 had a total activity of 30.84±5.14 U/ml, a protein content of 777.10±4.21 µg/ml, and a specific activity of 39.69±5.32 U/mg, as shown in table 2. Ammonium sulfate concentration increased protein content to 936.72±12.32 µg/ml and specific activity to 50.42±4.51 U/mg, with a purification fold of 1.27. After Sephadex G-100 purification, specific activity reached 92.83± 4.54 U/mg. For C. palmioleophila isolate JK12, the culture filtrate had 34.04±6.43 U/ml activity, 877.42± 13.68 µg/ml protein content, and 38.80±4.32 U/mg specific activity. After ammonium sulfate, specific activity increased to 47.83±3.22 U/mg (1.23 purification fold) and 76.0±3.71 U/mg after Sephadex G-100. Overall, T. asahii isolate ChL11 and C. palmioleophila isolate JK12 achieved purification folds of 2.34 and 2.28, respectively.
Physicochemical characterization of L-asparaginase: Determination of the molecular weight of the enzymes: SDS-PAGE analysis of L-ASNase from T. asahii isolate ChL11 (TaIChL11 L-ASNase) showed a single protein band after purification, with a molecular weight of around 40 kDa (Figure 1A), indicating successful isolation of the enzyme. Similarly, L-ASNase from C. palmioleophila isolate JK12 (CpIJK12 L-ASNase) showed a single band between 135 kDa and 180 kDa, matching the protein marker at approximately 135 kDa (Figure 1B). These results confirm the effective purification and consistent molecular weights of the L-ASNase enzymes from both isolates.
Effect of pH and temperature on L-asparaginase activity: The investigation into L-ASNase activity across different pH levels yielded significant findings. As shown in figure 2, L-ASNase from T. asahii isolate ChL11 initially showed an activity of 13.09±1.5 U/ml at pH 5.0. As the pH increased, the enzyme's activity also increased, reaching its maximum of 25.28±0.59 U/ml at pH=7.5. This represents a 193.13% increase, indicating that the enzyme functions optimally at a near-neutral pH. Beyond this peak, the activity declined, as shown in figure 2. Similarly, L-ASNase from C. palmioleophila isolate JK12 showed an initial activity of 11.23±0.77 U/ml at pH=5.0, peaking at 21.13±0.87 U/ml at pH=7.0, an enhancement of 188.16%, before gradually decreasing. These results highlight the complex relationship between pH and enzyme activity.
The study on L-ASNase activity examined the enzyme from 20°C to 45°C to find its optimal temperature. For L-ASNase from T. asahii isolate ChL11, the highest activity was at 35°C with 38.97±1.2 U/ml, a 204.03% increase from 20°C (19.1±1.2 U/ml), as shown in figure 3. Activity declined beyond 35°C, dropping to 15.35±2.06 U/ml at 45°C, retaining 80.36% of its initial activity at 20°C, indicating sensitivity to higher temperatures. Similarly, L-ASNase from C. palmioleophila isolate JK12 peaked at 35°C with 34.2±0.83, a 196.55% increase from 20°C (17.4±0.9 U/ml). Activity decreased past 35°C, reaching 17.65±0.43 U/ml at 45°C but retaining 101.44% of its activity at 20°C, showing notable temperature stability up to 45°C.
Effect of incubation time on L-asparaginase activity: Figure 4 illustrates the enzyme activity of TaIChL11 L-asparaginase and CpIJK12 L-asparaginase over a 40-min incubation period. In the initial phase (0–10 min), both enzymes show a slow increase in activity, with CpIJK12 L-ASNase showing a slightly faster reaction rate. Between 10 and 20 min, enzyme activity rises rapidly, likely due to faster substrate binding, indicating that both enzymes reach their optimal catalytic conditions. During this phase, TaIChL11 L-ASNase exhibits a steeper increase, surpassing CpIJK12 L-ASNase in activity. After 20 min, both enzymes approach their peak activity, stabilizing around 25–30 U/ml between 25 and 30 min, with TaIChL11 L-ASNase maintaining slightly higher enzymatic activity. These results indicate that both fungal L-asparaginases exhibit time-dependent enzymatic activity, with the most effective catalytic phase occurring between 10 and 20 min, followed by a plateau phase where activity stabilizes. This analysis highlights the dynamic nature of enzyme activities over time and underscores the differential kinetic properties of TaIChL11 L-ASNase and CpIJK12 L-ASNase.
Effect of substrate concentration on L-asparaginase activity: Examining kinetic parameters is pivotal in comprehending an enzyme's catalytic efficacy. In this study, the Michaelis-Menten constant (KM), representing the substrate concentration at which enzymes reach Vmax, along with Vmax and turnover number (Kcat) for both TaIChL11 L-ASNase and CpIJK12 L-ASNase were evaluated using Lineweaver-Burk plot, as shown in figures 5A and 5B. The KM of TaIChL11 L-ASNase and CpIJK12 L-ASNase was found to be 1.66×10-2 mM and 2.3×10-2 mM respectively, demonstrating a strong affinity of the enzymes towards their substrate, L-asparagine, as shown in table 3. The relatively low KM value suggests the enzymes’ effectiveness in converting substrate molecules into products even at low concentrations.
Furthermore, the Vmax of TaIChL11 L-ASNase and CpIJK12 L-ASNase was 37.23 mM/min and 14.03 µM/min respectively. These Vmax of the enzymes signify the maximum rate of the enzymatic reaction when the enzymes are saturated with substrate. This underscores their efficiency in catalyzing the conversion of L-asparagine. The turnover numbers (Kcat) for TaIChL11 and CpIJK12 L-ASNases were 3.7 and 1.4 min⁻¹, converting about 3.7 and 1.4 substrate molecules/min, respectively.
Substrate specificity of L-asparaginase: The purified fungal protein derived from the T. asahii isolate ChL11 revealed a significant contrast in enzymatic activity between L-ASNase and L-GLNase, as shown in table 4. Specifically, L-ASNase activity surpassed L-GLNase's by more than eightfold within the purified protein sample. This discrepancy highlights the considerable variation in enzymatic efficacy between these two functions within the fungal protein derived from T. asahii isolate ChL11. Similarly, the fungal protein obtained from the C. palmioleophila isolate JK12 upon purification exhibited a marked difference in enzymatic activity between L-ASNase and L-GLNase. The L-ASNase activity within the purified protein sample was more than six times higher than that of L-GLNase. This finding highlights the significant difference in enzymatic efficiency between the two activities of the fungal protein from C. palmioleophila isolate JK12.
The anti-proliferative potential of L-asparaginase: The study investigated the impact of L-asparaginases from T. asahii isolate ChL11 and C. palmioleophila isolate JK12 on CCRF-CEM cells, using the resazurin assay with commercial L-asparaginase as a reference standard. Results demonstrated that both fungal L-asparaginases exhibited dose-dependent and time-dependent inhibition of cell viability, indicating their potential as anticancer agents. Notably, higher concentrations of the fungal extracts (25 U/ml) significantly inhibited cell proliferation, while lower concentrations (0.78 U/ml) showed minimal effects. Furthermore, as depicted in figure 6, the antiproliferative activities of the L-ASNases increased with longer exposure times. Higher concentrations of both fungal L-ASNases were notably more effective at inhibiting cell proliferation, underscoring their potential as anticancer agents at adequate doses. Additionally, longer exposure times resulted in greater inhibition of cell viability, suggesting that extended treatment with these enzymes could enhance their effectiveness in a therapeutic setting.
The assessment of fungal-derived L-ASNases on the CCRF-CEM leukemia cell line revealed significant growth inhibitory effects, as shown in figure 7. The IC50 values (enzyme concentration required to inhibit 50% of cell proliferation) varied based on the enzyme source and exposure duration. For TaIChL11 L-ASNase, the IC50 values were 3.62±0.05, 2.74±0.01, and 1.60±0.45 U/ml after 24, 48, and 72 hr, respectively. Similarly, CpIJK12 L-ASNase exhibited IC50 values of 4.91±0.06, 3.30±0.23, and 1.91±0.38 U/ml over the same periods, respectively. These findings suggest that TaIChL11 L-ASNase offers superior efficacy in inhibiting CCRF-CEM cell proliferation, highlighting its potential for therapeutic applications in cancer treatment. However, both fungal L-ASNases exhibited significantly higher IC50 values compared to commercial L-ASNase, which demonstrated the lowest IC50 values at all exposure times (3.32±0.46, 2.35±0.005, and 1.29±0.54 U/ml, respectively), indicating its superior potency. The bar graph in figure 7 illustrates this trend, showing that at 24 hr, CpIJK12 L-ASNase had the highest IC50 value, suggesting the least potent inhibition, while TaIChL11 L-ASNase exhibited a lower IC50 value, indicating greater efficacy. Over 48 hr, all IC50 values decreased, reflecting increased enzyme effectiveness with prolonged exposure, with TaIChL11 L-ASNase maintaining superior inhibition compared to CpIJK12 L-ASNase. By 72 hr, the IC50 values further declined, demonstrating a time-dependent increase in cytotoxicity. While commercial L-ASNase consistently exhibited the lowest IC50 values, fungal L-ASNases still showed notable anti-proliferative activity, with TaIChL11 L-ASNase proving more effective than CpIJK12 L-ASNase. These results suggest that, despite the higher potency of commercial L-ASNase, TaIChL11 L-ASNase could be a promising alternative f or therapeutic use.
Determination of the cytotoxic concentration of L-asparaginase: In assessing the cytotoxicity of L-ASNase against the Vero E6 cell line over a 24 hr exposure period, the concentrations required to achieve 50% cytotoxicity were 12.08±5.26 U/mL for TaIChL11 L-ASNase, 16.67±7.92 U/ml for CpIJK12 L-ASNase, and 9.99±5.37 U/ml for commercial L-ASNase. For a 48 hr exposure time, the CC50 values were 10.94±3.26, 13.14±5.04, and 7.32±1.75 U/ml, respectively for TaIChL11L-ASNase, CpIJK12 L-ASNase, and commercial L-ASNase. Additionally, for a 72 hr exposure period, the CC50 values further decreased to 5.45±0.12 for TaIChL11 L-ASNase, 5.45±0.12 for CpIJK12 L-ASNase, and 4.25±0.38 U/ml for commercial L-ASNase, respectively as shown in figure 8 and table 5. These concentrations reflect the levels at which L-ASNase shows at least 50% cytotoxicity in Vero E6 cells.
Determination of the selectivity index of L-asparaginase: The relative safety margin of L-ASNase, indicated by the drug Selectivity Index (SI), was calculated by dividing the CC50 value by the IC50 value. For a 24 hr exposure period, the SI values revealed that the concentration required to inhibit 50% of cancer cell proliferation was significantly higher than that needed to achieve the same effect on normal cells: 3.34 for TaIChL11 L-ASNase, 3.40 for CpIJK12 L-ASNase, and 3.01 for commercial L-ASNase. Similarly, these values increased over a 48 hr exposure period to 3.99, 3.98, and 3.11, respectively. Additionally, for 72 hr exposure period, the SI values were 3.41 for TaIChL11 L-ASNase, 2.82 for CpIJK12 L-ASNase, and 3.29 for commercial L-ASNase, as shown in table 5. Furthermore, the SI values in the present study indicate that greater selectivity for both fungal L-ASNases was observed after 48 hr of exposure. On the other hand, the maximum SI for commercial L-ASNase was recorded after 72 hr of exposure. In general, these SI values indicate that the cancer cells were more sensitive to L-ASNases compared to the normal cells.
Evaluating IgG and IgE production for L-ASNase hypersensitivity in mice: To examine the immunogenicity of L-ASNase, an in vivo experiment was conducted with four groups of mice. During the initial sensitization period (days 1-13), the treatment groups received 56.25 U/kg of L-ASNase derived from T. asahii isolate ChL11, C. palmioleophila isolate JK12, or commercial L-ASNase. Unlike the negative control group, these groups showed an immune response, which did not receive L-ASNase. In the subsequent sensitization phase (days 14-23), the dose was increased to 112.5 U/kg. All L-ASNase-treated groups exhibited significant increases in serum IgG levels compared to the negative control group. Notably, mice treated with commercial L-ASNase had higher IgG titers than those treated with L-ASNase from T. asahii isolate ChL11 or C. palmioleophila isolate JK12.
Furthermore, when the dose was increased to 1125 U/kg, the immune response (IgG level) in mice treated with L-ASNase derived from T. asahii isolate ChL11 was significantly lower than in those treated with commercial L-ASNase. However, there was no significant difference in IgG levels between mice treated with commercial L-ASNase and those treated with L-ASNase derived from C. palmioleophila isolate JK12. This suggests that while all forms of L-ASNase can induce an immune response, the commercial version and the one derived from C. palmioleophila isolate JK12 elicit a stronger reaction, particularly at higher doses, as depicted in figure 9A. In assessing the production of anti-L-ASNase, IgE in mice treated with TaIChL11 L-ASNase, CpIJK12 L-ASNase, and commercial L-ASNase, the results showed no significant immune response against any of the three types compared to the negative control group. This indicates that the mice did not produce notable levels of IgE antibodies in reaction to these L-ASNases as depicted in figure 9B.
Discussion :
Purifying enzymes is essential for removing contaminants and enhancing specificity, efficacy, and stability 22. In this study, L-ASNase from T. asahii isolate ChL11 and C. palmioleophila isolate JK12 was purified through multiple steps, revealing variations in protein content, enzyme activity, and specific activity, which reflect the purification process's effectiveness. As expected, higher purity led to an increased specific activity, indicating a more concentrated and potent enzyme 42. Determining enzyme molecular weight is also crucial for understanding function, guiding characterization, and ensuring purity, which informs purification strategies and quality control in biomedical and industrial applications 43. SDS-PAGE analysis confirmed that L-ASNase from T. asahii isolate ChL11 had a molecular weight of approximately 40 kDa, while the enzyme from C. palmioleophila isolate JK12 had a molecular weight of 135 kDa, consistent with with L-ASNases from E. coli (138-141 kDa), Erwinia (138 kDa), and Rhizomucor miehei (133.7 kDa 44-47. In contrast, L-ASNases purified from other micro-organisms, like Lasiodiplodia theobromae and C. utilis, exhibit varying molecular weights (70 kDa and 75 kDa, respectively) 12,48, likely due to genetic differences among the organisms.
Temperature and pH play a crucial role in determining enzyme efficiency by influencing the structural integrity of the active site and the overall reaction rate 22. Optimizing these factors is key for enhancing L-ASNase activity 23. In this study, L-ASNase from T. asahii isolate ChL11 showed optimal activity at pH 7.5, while C. palmioleophila isolate JK12 peaked at pH=7.0. Both enzymes were most active at 35°C and remained stable between 20°C and 45°C, highlighting their broad functionality. These findings align with other studies 49,50 but also reveal varying optimal conditions among different microbial sources 51, emphasizing the diversity in L-ASNase characteristics.
Understanding enzyme kinetics and substrate specificity is key to optimizing efficacy and reducing side effects. This study investigates how different substrate concentrations impact L-ASNase activity and its specificity. The KM for TaIChL11 and CpIJK12 L-ASNases were 1.66×10⁻² and 2.3×10⁻² mM, respectively. This finding aligns with the results reported by 52, wherein L-ASNase extracted from Penicillium digitatum (P. digitatum) showed a KM of 1.0×10-2 mM which is also similar to the two main currently marketed therapeutics, Oncaspar® and Erwinase®, enzymes that have KM values of approximately 0.02–0.05 and 0.05 mM, respectively 53,54. This indicates the enzymes identified in the present study have strong substrate affinity and effective conversion even at low concentrations. Moreover, the enzymes found in the current study found Vmax values of 37.23 mM/min for TaIChL11 L-ASNase and 14.03 mM/min for CpIJK12 L-ASNase, which are lower compared to Lasiodiplodia theobromae (127 μM ml-1 min-1) and Streptomyces fradiae NEAE-82 (95.08 U/ml/min) 48,50.
L-asparaginase (L-ASNase) is a crucial enzyme in leukemia treatment, depleting asparagine to inhibit cancer cell proliferation. While bacterial L-ASNases are commonly used, their immunogenicity and side effects necessitate alternative sources. This study evaluated the anticancer potential of two fungal-derived L-ASNases, TaIChL11 and CpIJK12, using CCRF-CEM leukemia cells, with commercial L-ASNase as a reference. Both fungal enzymes exhibited significant dose- and time-dependent antiproliferative effects, effectively inhibiting cell viability even at low concentrations. These findings highlight the potential of fungal L-ASNases as alternative therapeutic agents for leukemia treatment. TaIChL11 L-ASNase showed increasing potency over time, with IC50 values decreasing from 3.62±0.05 at 24 hr to 1.60±0.45 U/ml at 72 hr. In comparison, CpIJK12 L-ASNase had IC50 values of 4.91±0.06 at 24 hr, 3.30±0.23 at 48 hr, and 1.91±0.38 U/ml at 72 hr. These values are higher than those reported for other L-ASNases, such as 1.5 U/ml after 72 hr 55, 1 U/ml after 24 hr 56, 0.22 U/ml after 48 hr for Aspergillus niger (A. niger) AKV-MKBU, 2.0 U/ml for Trichoderma koningii, and 2.5 U/ml for Trichoderma iride HK01 after 24 hr 57,58. In contrast, L-ASNase from Aspergillus sydowii (A. sydowii) and Fusarium oxysporum (F. oxysporum) had IC50 values of 50.0 and 62.5 U/ml, respectively 59, while L-ASNase from Lasiodiplodia theobromae showed an IC50 of 35.2±0.7 U/ml 48. These comparisons highlight the diverse potency of L-ASNases and their potential for cancer treatment.
The cytotoxicity assessment on Vero E6 cells showed that TaIChL11 L-ASNase had CC50 values of 12.08±5.26 at 24 hr, 10.94±3.26 at 48 hr, and 5.45±0.12 U/ml at 72 hr, while CpIJK12 L-ASNase had CC50 values of 16.67±7.92 U/ml at 24 hr, 13.14±5.04 U/ml at 48 hr, and 5.39±0.001 U/ml at 72 hr. These results indicate that TaIChL11 and CpIJK12 L-ASNases have higher CC50 values, suggesting lower cytotoxicity compared to the 8.5±0.5 U/ml CC50 of Streptomyces rochei L-ASNase against WI-38 cells after 24 hr 60. In contrast, these values are much lower than the 79.4±1.9 U/ml for L-ASNase from Lasiodiplodia theobromae, indicating higher cytotoxicity 48. The variability in cytotoxicity can be attributed to differences in the source organism, enzyme purification levels, and the sensitivity of cell lines, with fungal L-ASNases potentially undergoing unique post-translational modifications or structural changes compared to bacterial ones.
Type I and Type II hypersensitivity reactions are immune responses triggered by antibodies. Type I hypersensitivity is mediated by immunoglobulin E (IgE) and is associated with allergic reactions 61. In contrast, Type II hypersensitivity involves immunoglobulin G (IgG) 62. This study examined anti-L-ASNase IgG and IgE to assess the potential immune reactions to L-ASNase. During initial sensitization (days 1-13), IgG levels were similar across all L-ASNases, indicating equal immunogenicity likely due to the low dosage. However, with repeated exposure, mice showed higher IgG levels to commercial and CpIJK12 L-ASNase than to TaIChL11 L-ASNase, highlighting variability in immunogenicity depending on the enzyme source. These findings align with bioinformatics data indicating that fungal L-ASNases have lower immunogenicity than bacterial ones 63. Moreover, this study found no significant IgE production in any treatment group, including commercial and fungal L-ASNases, with IgE levels similar to the negative control. This suggests that neither form induced IgE-mediated immune responses, contrasting with previous reports of L-ASNase sensitization triggering IgE production 64. The contradiction with previous studies may be due to structural differences in L-ASNases, which could affect the enzyme's immunogenicity and result in varying levels of IgE-mediated hypersensitivity.
Conclusion :
The SDS-PAGE analysis confirmed the successful purification of L-ASNase from T. asahii isolate ChL11 (TaIChL11 L-asparaginase) and C. palmioleophila isolate JK12 (CpIJK12 L-asparaginase). The low Km values and high Vmax highlight their efficiency in substrate conversion. Moreover, their significantly higher L-ASNase activity than L-GLNase indicates their selectivity and specificity to L-asparagine which could reduce side effects associated with the enzymes’ L-GLNase co-activity. Both TaIChL11 L-ASNase and CpIJK12 L-ASNase selectively target cancer cell proliferation while sparing normal cells. The comparable antiproliferative potential of fungal L-asparaginases to their bacterial counterparts underscores their promise as effective therapeutic agents for treating cancer, particularly ALL. The lower production of IgG against TaIChL11 L-ASNase compared to commercial L-ASNase, along with the absence of IgE production, underscores the enzyme's favorable safety profile. In conclusion, these results suggest that fungal L-ASNases from T. asahii and C. palmioleophila have strong potential as therapeutic agents.
Ethical approval :
Before starting the study, ethical approval was obtained from Mount Kenya University's Research Ethics Committee (MKU/ISERC/2949).
Acknowledgement :
The African Union Commission (AU) deserves credit for supporting this study through the Pan African University Institute of Basic Sciences, Technology, and Innovation (PAUSTI). We are deeply grateful to Kenya Medical Research Institute (KEMRI) for providing access to their cell culture laboratory.
Moreover, we would like to express my sincere gratitude to Mount Kenya University's Research Ethics Committee for granting ethical approval for this study (Approval No. MKU/ISERC/2949). Their support and guidance have been invaluable throughout this research.
Conflict of Interest :
This statement confirms that all authors have declared no conflicts of interest.
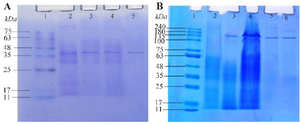
Figure 1. SDS-PAGE gel images of L-asparaginases. Figure 1A shows an SDS-PAGE gel image of L-asparaginase from T. asahii isolate ChL11. Lane 1 represents the marker, Lane 2 the culture filtrate, Lane 3 the ammonium sulfate fraction, Lane 4 the dialyzed enzyme, and Lane 5 the purified enzyme using a Sephadex G-100 column. Similarly, Figure 1B displays the SDS-PAGE gel image of L-asparaginase from C. palmioleophila isolate JK12, with Lane 1 being the protein marker, Lane 2 the dialyzed enzyme, Lane 3 the ammonium sulfate fraction, Lane 4 the culture filtrate, and Lane 5 and 6 the purified enzyme using a Sephadex G-100 column.
|
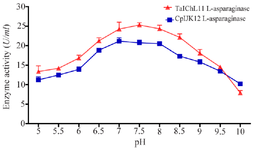
Figure 2. Effect of pH on L-ASNase activity.
|
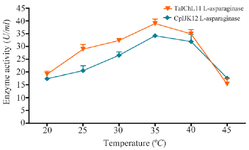
Figure 3. Effect of temperature on L-ASNase activity.
|
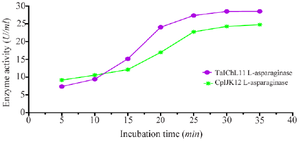
Figure 4. Effect of incubation time on L-asparaginase activity.
|

Table 5. L-asparaginase SI evaluated on normal and cancer cells
Note: The mean IC50 and CC50 values were derived from two independent experiments, as presented in Figures 6 (A-C) and 8 (A-C), with the corresponding IC50 and CC50 data provided in Appendix 2.2.
|
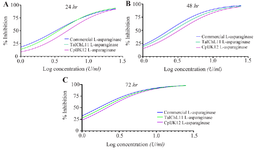
Figure 6. Antiproliferative activity of L-ASNases on CCRF-CEM cells over different treatment periods. Antiproliferative activity of TaI11ChL L-asparaginase, CpIJK12 L-asparaginase, and commercial L-ASNase on CCRF-CEM leukemia cells over 24 (Figure A), 48 (Figure B), and 72 hours (Figure C) of incubation. The values presented are the mean of three independent experiments (n=3). The coefficient of determination (R2) was above 0.96 for all the curves.
|
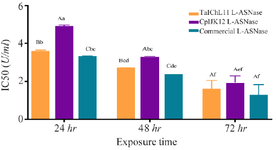
Figure 7. Comparison of the antiproliferative activity of TaIChL11 L-asparaginase, CpIJK12 L-asparaginase, and commercially sourced L-ASNase against CCRF-CEM cell line. Different IC50 values for each tested extract were plotted against the enzyme exposure time. Bars sharing the same lowercase letter (a-f) are not significantly different across all bars, and bars sharing the same uppercase letter (A-C) are not significantly different within the same exposure time (p>0.05; one-way ANOVA followed by Tukey’s HSD test).
|
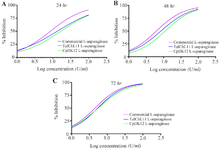
Figure 8. Cytotoxic activity of TaIChL11 L-ASNase, CpIJK12 L-ASNase, and commercially sourced L-ASNase against Vero E6 cells. Cytotoxic activity of TaIChL11 L-ASNase, CpIJK12 L-ASNase, and commercially sourced L-ASNase against Vero E6 cell line over 24 (Figure A), 48 (Figure B), and 72 hr (Figure C) of incubation. Values represent the mean of three independent experiments (n=3), with an R² above 0.96 for all curves.
|

Figure 9. Time course of anti-L-asparaginase IgG (Figure A) and anti-L-asparaginase IgE (Figure B) development in the serum of mice. This figure shows the immunogenic response in Balb/c mice treated with commercial L-asparaginase (positive control), TaIChL11 L-ASNase, CpIJK12 L-ASNase, or saline (negative control) on days 1-13, 14-23, and 24-28. Figure A displays serum IgG levels, while Figure B shows serum IgE levels, with data as mean±SD (ng/ml) over time. In Figure A, bars with the same lowercase letter (a-f) show no significant difference across all groups, and bars with the same uppercase letter (A-D) show no difference at the same time point (one-way ANOVA with Dunnett’s test, p>0.05). No significant differences were observed in Figure B, indicating no letters.
|

Table 1. Optimization of L-ASNase production condition on MCD media using fungal isolates
|
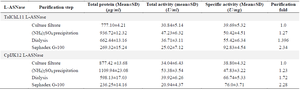
Table 2. Purification profile of L-asparaginase from T. asahii isolate ChL11 and C. palmioleophila isolate JK12
|

Table 3. L-asparaginase kinetic parameters
|

Table 4. Comparative analysis of purified fungal enzymes' L-ASNase and L-GLNase activities
|
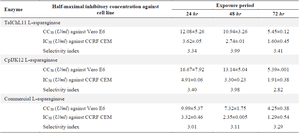
Table 5. L-asparaginase SI evaluated on normal and cancer cells
Note: The mean IC50 and CC50 values were derived from two independent experiments, as presented in Figures 6 (A-C) and 8 (A-C), with the corresponding IC50 and CC50 data provided in Appendix 2.2.
|
|