Production and Characterization of IgY Polyclonal Antibodies Specific to Human Interleukin-6 and Their Neutralization Potential for Anti-inflammatory Responses
-
Khumpim, Pacharaporn
-
Institute of Molecular Biosciences, Mahidol University, Salaya, Phutthamonthon, Nakhon Pathom, Thailand
-
Faculty of Veterinary Science, Mahidol University, Salaya, Phutthamonthon, Nakhon Pathom, Thailand
-
Thawornkuno, Charin
-
Faculty of Tropical Medicine, Mahidol University, Ratchathewi, Bangkok, Thailand
-
Piboonpocanun, Surapon
-
Institute of Molecular Biosciences, Mahidol University, Salaya, Phutthamonthon, Nakhon Pathom, Thailand
-
Wongwadhunyoo, Wongsakorn
-
Faculty of Veterinary Science, Mahidol University, Salaya, Phutthamonthon, Nakhon Pathom, Thailand
-
 Masrinoul, Promsin
Institute of Molecular Biosciences, Mahidol University, Salaya, Phutthamonthon, Nakhon Pathom, Thailand, E-mail: promsin.mas@mahidol.edu
Masrinoul, Promsin
Institute of Molecular Biosciences, Mahidol University, Salaya, Phutthamonthon, Nakhon Pathom, Thailand, E-mail: promsin.mas@mahidol.edu
Abstract: Background: Interleukin-6 plays an essential role in cytokine storm and cytokine release syndrome, which occur in response to pathogen infection or tissue injury and are associated with severe symptoms. Neutralizing IL-6 can help reduce symptom severity. Chicken eggs serve as an excellent alternative antibody source compared to mammalian serum. The immunoglobulin Y (IgY) in the chicken’s blood is transferred to and deposited within the egg yolk in large amounts. Several IgY products have been developed for therapeutic applications in various diseases. This study focuses on producing anti-human IL-6 (IL-6) IgY antibodies to support therapeutic advancements.
Methods: Anti-IL-6 IgY was generated by immunizing three hens with recombinant IL-6 protein mixed with alum adjuvant, immunized four times at three-week intervals. Then, IgY was extracted from egg yolks. The specificity of IgY was determined by Western blot. The neutralizing activity against secreted IL-6 was demonstrated by Human Coronavirus OC43 (HCoV-OC43)-infected cells and Lipopolysaccharide (LPS)-stimulated human lung fibroblast MRC-5 cells.
Results: The specific anti-IL-6 was detected starting from day 10 to day 90 after immunization. The average yield of total IgY was 17.97±15.66 mg per egg. The extracted anti-IL6 IgY antibody exhibited efficient neutralizing effects against secreted IL-6 in the HCoV-OC43-infected or LPS-stimulated cells in a dose-dependent manner.
Conclusion: This study highlights the ease of production and the satisfactory yield of anti-IL-6 IgY derived from chicken eggs. The antibody demonstrates an in vitro inhibitory effect on IL-6, with potential applications in therapeutic development.
Introduction :
Elevated levels of interleukin-6 (IL-6) are associated with worse outcomes in various infectious diseases, for example, severe human coronavirus disease 2019 (COVID-19), enterovirus71 (EV71) which causes encephalitis, coxsackievirus A16 causing hand, foot and mouth disease 1-3. In severe COVID-19 case, the immune dysregulation results in the overproduction of several pro-inflammatory cytokines including IL-6, interleukin-17 (IL-17), and Tumor Necrosis Factor-alpha (TNF-α), leading to a phenomenon known as a cytokine storm and cytokine release syndrome. This excessive cytokine production contributes to organ damage and can be life-threatening for patients. In addition to viral infections, high levels of IL-6 are ob-served in bacterial sepsis, often associated with tissue damage, organ failure in severe cases 4,5. This indicates that IL-6 is the key cytokine driving disease progression in both viral and bacterial infection.
Human IL-6 is a 22 kDa protein containing 212 amino acids, formerly known as a B-cell differentiation factor. IL-6 is produced in response to various factors such as tissue damage, infection, fever, and acute phase responses 6-10. In the immune system, macrophages are a major source of IL-6, which is secreted when Pathogen-Associated Molecular Patterns (PAMPs) interact with Pattern Recognition Receptors (PRRs) including Toll-Like Receptors (TLRs) of macrophages. Besides macrophages, other cells such as mast cells, dendritic cells, monocytes, and T cell and B cells, fibroblasts and endothelial cells also produce IL-6. When IL-6 binds to its receptor (IL-6R), it has dual functions, acting as both a proinflammatory and anti-inflammatory mediator. IL-6R exists in two forms: a membrane-bound form, known as the intramembranous IL-6 receptor, which is expressed on specific cell types such as monocytes, macrophages, neutrophils, hepatocytes, and some lymphocytes, and a soluble form (sIL-6R) that regulates the trans-signaling pathway. The intramembranous IL-6R can be cleaved by the ADAM-17 protein to generate sIL-6R, shifting the signaling mechanism from classical signaling to the trans-signaling pathway. The signal transduction of IL-6 begins when an IL-6 molecule attaches to IL-6R, either in its intramembranous or soluble form. The complex subsequently associates with membrane bound gp130 to initiate the downstream IL-6 signaling such as JAK/STAT cascade, which activates NF-κB mediated transcription of IL-6 mRNA translation 11. In humans, all cell types express gp130 on their membranes but only certain cells express IL-6R. Cells lacking IL-6R on their surface cannot directly respond to IL-6 stimuli directly. However, IL6 can exert effects on the IL-6R negative cells through the trans-signaling pathway, where the IL-6/sIL-6R complex binds to gp130 and stimulates signal transduction. This mechanism plays an important role during inflammation especially in cytokine storm and cytokine release syndrome which worsen the illness condition 12.
Therefore, blocking the IL-6 signal transduction pathway is a promising strategy to ameliorate severe symptoms 10. Several IL-6R antibody blocker antibodies, for example, Tocilizumab, have been reported as effective therapeutic drugs of COVID-19 with a hyperinflammatory condition and cytokine release syndrome as well as for treatment of various autoimmune and inflammatory conditions especially rheumatoid arthritis 10,13. Other IL6R blockers, Sarilumab and Satralizumab, are recommended for treating rheumatoid arthritis and neuromyelitis optica spectrum disorder, respectively 14. In addition to IL-6R blockers, several anti-IL-6 monoclonal antibodies have been intensively developed for targeted therapy of many diseases in both preclinical and clinical studies. Examples of the IL-6 antagonists include Olokizumab, Sirukumab, Siltuximab, Clazakizumab, PF-423691, and MEDI5117. These have been studied for their potential as IL-6-targeted immunotherapies for conditions such as rheumatoid arthritis, cutaneous and systemic lupus erythematosus, Castleman disease, multiple myeloma, prostate cancer, and renal cell carcinoma 15. In the case of virus infection, treatment with an anti-IL6 antibody to treat EV71 infected mice showed decreased tissue damage, increased activation of immune cells, and higher IL-10 level without affecting on viral load 1. In chronic inflammation and autoimmune diseases, the long-term continuous dysregulation of IL-6 expression occurs, which can worsen the disease conditions due to systemic biological dysfunction. For that reason, IL-6-based therapies using anti-IL6 and anti-IL-6R antibodies have been developed and proven to be successful therapeutics for many diseases and cancers 6.
Therapeutic treatments are not limited to monoclonal antibodies but also include polyclonal antibodies, such as rabies immunoglobulin, diphtheria antitoxin, and tetanus antitoxin. Until now, there has been no commercial polyclonal antibody against human IL-6. Most therapeutic polyclonal antibodies are traditionally produced from the serum of immunized horses and healthy human donors 16. However, this process, particularly in animal production is invasive and requires good husbandry practices to ensure animal welfare and the quality of the antibodies produced. As an alternative, producing antibodies using chickens is an increasingly attractive approach, particularly from an ethical perspective. Chickens naturally transfer antibodies, defined as the immunoglobulin Y (IgY), to their offspring by mainly depositing them into the egg yolk. This makes it convenient to harvest antibodies directly from the eggs, offering a noninvasive and humane method of polyclonal IgY antibody production 17,18. This technique minimizes pain and stress for animals during sample collection and aligns with ethical principles, including the refinement and reduction aspects of animal welfare policies 19-21.
Moreover, IgY production offers several advantages compared to traditional IgG production. IgY is more stable 22 and does not interact with complement components or Fc receptors, making it particularly beneficial for therapeutic applications in humans 23. It also allows for high-yield production and is scalable to an industrial level 24. Furthermore, purifying antibodies from egg yolk is relatively simple, cost-effective, and less time-consuming compared to purification from mammalian serum 17. Therefore, this study aimed to produce an anti-IL-6 polyclonal IgY antibody from chicken eggs. The immunization protocol in laying hens was conducted and subsequently the anti-IL-6 IgY was isolated and extracted from egg yolk. The quantity of the antibody was determined by ELISA and the specificity and biological activity of anti-IL6 were evaluated by Western blot and an in vitro neutralization assay using Human Coronavirus OC43 (HCoV-OC43)-infected cells and Lipopolysaccharide (LPS)-induced inflammation models.
Materials and Methods :
Immunization of chicken and egg collection: The animal experiment was approved by Faculty of Veterinary Science, Mahidol University Institute of Animal Care and Use Committee (MUVS-IACUC COA No. MUVS 2022-02-07), Three laying hens (Hy-Line Brown) aged 4-5 months and weight of >1,450 g were housed under standard conditions and acclimatized for 7 days before immunization. The hens were allocated to individual cages and fed a commercial layer diet and water ad libitum. Recombinant IL-6 (rIL-6, active protein) for immunization was purchased from MyBiosource (USA). Each hen was immunized at 3-week intervals with 100 μg of rIL-6 mixed with Alhydrogel® (aluminum hydroxide gel) adjuvant (InvivoGen) at day 0, 21, 42, and 63 via intramuscular injection at two sites of pectoral muscle (100 µl per site of injection). The eggs were collected daily from day 0 until day 119, labeled and stored in 4°C until the next step.
Total IgY extraction: Eggs were collected from laying hen in both pre-immunized eggs and immunized eggs. The IgY was extracted from egg yolk individually by ammonium sulfate precipitation. Briefly, egg yolk was separated and diluted in 10 volumes of cold acidic distilled water (pH=5). The pH of the suspension was adjusted to 5.0 using 1 M HCl. The suspension was incubated at 4℃ for 2 hr, centrifuged at 10,000×g at 4℃ for 30 min and the supernatant was collected. The supernatant was mixed with a saturated ammonium sulfate solution to a final concentration of 40%. The suspension was incubated at 4℃ for 20 min, then centrifuged at 10,000×g at 4℃ for 30 min and the pellet was retained. The pellet was dissolved in 5 ml Phosphate-Buffered Saline (PBS) and the purity of IgY was assessed by sodium dodecyl sulfate polyacrylamide (SDS-PAGE) gel electrophoresis under non-reducing conditions. Briefly, 2 µg of each extracted IgY was loaded in SDS-PAGE buffer without β-mercaptoethanol. The samples were run on 8% SDS-PAGE gel at 100 V for 60 min. The gel was strained with Coomassie Brilliant Blue (Bio-Rad) and destained with a solution of 40% methanol and 10% acetic acid. The purity of IgY was analyzed using Image J V.154k and interpreted as % purity relative to impurity bands. The specificity was determined by Western blot. Extracted IgYs were stored at -20°C until use.
Characterization of IgY anti-human IL-6 antibody: Western blot analysis: Two mg of rIL-6 was denatured in SDS-PAGE buffer (125 mM Tris-HCl pH=6.8, 4% SDS, 20% glycerol, 4% β-mercaptoethanol and 0.02% bromophenol blue) at 95℃ for 5 min before running electrophoresis in a 12.5% SDS-PAGE gel at 75 V for 90 min. The rIL-6 in the gel was electrophoretically transferred onto a nitrocellulose membrane. The membrane was submerged in phosphate buffered saline solution A (PBS-A: 10 mM phosphate, 137 mM NaCl, 2.7 mM KCl, 0.1% Tween-20 and 3% non-fat dry milk) for 1 hr before being incubated with IgY and a commercial mouse anti-IL6 antibody at a dilution of 1:1000 at 4°C overnight. The membrane was washed before incubated with Horseradish Peroxidase (HRP) conjugated goat anti-chicken IgY antibody at dilution 1:5000 (Invitrogen, USA) or HRP conjugated goat anti-mouse IgG at a dilution of 1:5000 (Invitrogen, USA) at Room Temperature (RT) for 90 min. The membrane was washed before being incubated with Luminata™ Forte Western HRP substrate, and the emitted signal was captured by the AzureTM imager.
Enzyme-Linked Immunosorbent Assay (ELISA): Titers of anti-IL6 IgY were determined by indirect ELISA. Briefly, 500 ng of rIL-6 in PBS was added to each well of a 96-well microplate and incubated at 4℃ overnight. The plate was then washed and blocked with a blocking reagent (3% skim milk in PBS and 0.05% Tween-20) for 1 hr at RT. The plate was washed and incubated for 2 hr at RT with 10-fold serial dilution of extracted anti-IL-6 IgY or blocking reagent, used as a negative control. Following incubation, the plate was washed and then incubated for 1 hr at RT with an HRP-conjugated goat anti-chicken IgY antibody at a dilution of 1:5000 (Invitrogen, USA). After washing, 3,3',5,5'-Tetramethylbenzidine (TMB) substrate was added and incubated at RT for 15 min. The reaction was then stopped by adding 2 M sulfuric acid. Color intensity of the reaction was measured as the absorbance value at 450 nm. A signal-to-cutoff (S/Co) value was calculated by subtracting the OD of the negative control from the sample OD. Samples with an S/Co value ≥1 were identified as positive. The results were reported as the endpoint antibody titer of the total log IgY titer.
In vitro IL6 neutralization assay: Human coronavirus OC43 virus infected cell induced IL-6 production: The human lung fibroblast cell line MRC-5 (CCL-171, ATCC, USA) infected with human coronavirus OC43 (HCoV-OC43) (VR1558, ATCC, USA) was used to study the neutralization activity of anti-IL-6 IgY on the biological functions of secreted IL-6 from virus-infected cells. The HCoV-OC43 virus was grown and propagated in MRC-5 cells cultured in MEM medium containing 10% Fetal Bovine Serum (FBS) to prepare virus stock. MRC-5 cells (1×10⁷ cells) were infected with HCoV-OC43 at a multiplicity of infection (MOI) of 0.1 for 1 hr at 33°C in a T25 culture flask. The infection was performed in triplicate. The infected and non-infected cells were cultured in MEM medium containing 2% FBS at 33°C with 5% CO2. Then, anti-IL-6 IgY was added at the indicated concentrations (0, 25, 50, 100, and 200 µg) at day 3 of the experiment. The supernatant of each flask was collected every day for 5 days after viral infection to measure the amount of secreted IL-6 and to determine the virus titer by the TCID50 assay 25.
The amount of secreted IL-6 in the collected medium was measured according to the protocol of the Human IL-6 Uncoated ELISA kit (Invitrogen, USA) provided by the manufacturer. Briefly, the anti-IL6 capture antibody was coated in each well of a 96-well plate and incubated at 4℃ overnight. The plate was washed and incubated with blocking buffer (ELISA/ELISPOT Diluent) at RT for 1 hr. The plate was washed and incubated with a biotin-conjugated IgG anti-IL6 antibody at RT for 2 hr. The plate was washed and incubated with HRP-conjugated Streptavidin at RT for 30 min. TMB substrate was added and incubated at RT for 15 min, then a stop solution (2 M sulfuric acid) was added. Color intensity was measured as absorbance at 450 nm. Samples with a sample-to-positive control (S/P) ratio values ≥1 were identified as a positive reaction. Secreted IL-6 levels were calculated according to the standard curve generated by the Human IL-6 Uncoated ELISA kit (Invitrogen, USA).
LPS-induced IL-6 production: MRC-5 cells (1×10⁷ cells) were cultured in a T25 culture flask using MEM medium containing 10% FBS at 37°C in 5% CO2. The monolayers of MRC-5 cells were treated with 1 µg of LPS in triplicate. Then, the anti-IL-6 IgY was added at the indicated concentrations (0, 25, 50, 100 and 200 µg) at day 3 of the experiment. The supernatant of each flask was collected daily to measure IL-6 levels using the human IL-6 ELISA kit (Invitrogen, USA).
Data analysis: For antibody production, the IgY antibody titers were measured at several time points to evaluate the IgY production protocol in laying hens. The levels of antibodies were analyzed using descriptive statistics. Statistical analysis of the neutralizing activity of IgY against IL-6 in vitro was analyzed using Student’s t-test comparing to its pre-immune IgY antibody level. The differences were considered significant when the p value was <0.05.
Results :
Production of anti-human IL-6 IgY in laying hen: For the generation of anti-IL6 IgY antibodies, three laying hens (Hy-Line Brown) were intramuscularly immunized with rIL-6 four times, with 3-week intervals. The eggs from the immunized hens were collected every day. The total IgY antibodies were extracted from the egg yolk of individual eggs by ammonium sulfate precipitation. The purity of IgY was determined by SDS-PAGE analysis, as shown representatively in figure 1A. The purity of the extracted samples varied, with the average purity of anti-IL-6 IgY being 66.11±20.49. The ELISA results showed that after immunization, the specific anti-IL6 IgY rose at an early stage by ten days after the initial immunization with the average of Log10 IgY titer against IL-6 being 4.204±0.521. Interestingly, the level of antibody titers was maintained within the range of 3.702±0.348 and 4.404±0.173 throughout production (Figure 2).
The extracted IL-6 IgY antibody was specific to human IL-6: IgY extracted at 10, 30, 60, and 90 days post-immunization (dpi) was selected to determine its specific binding activity by Western blot. In this experiment, the rIL6 protein was loaded into an SDSPAGE gel and transferred to a nitrocellulose membrane. The membrane was incubated with selected extracted IgY of pre-immune, day 10, 30, 60, and 90 post-immunization or commercial anti-IL6 mouse antibody as the control. The membranes were then stained with an anti-chicken IgY conjugated HRP or anti-mouse IgG conjugated HRP. The specific signal was captured by an imager after adding the HRP substrate. The results showed that anti-IL-6 IgY specifically bound to the rIL-6 protein, with no immunogenic reaction observed when using the pre-immune IgY antibody (Figure 1). These results indicate the specificity of the extracted IgY for human IL-6 and support the rapid production of specific anti-IL-6 antibodies following immunization.
The anti-human IL-6 IgY neutralize human IL-6 in vitro systems: The functional activity of anti-IL-6 IgY to neutralize human IL-6 was demonstrated in vitro using HCoV-OC43-infected cells and LPS-induced IL-6 production models. As mentioned, patients infected with coronaviruses exhibit excessive IL-6 stimulation. In this study, the neutralizing activity of anti-IL-6 IgY using human lung fibroblast MRC-5 cells infected with the HCoV-OC43 virus was tested. The results showed that after viral infection, IL-6 was secreted into the culture supernatant by day 2 post-infection, with levels gradually increasing to 2,695.06±128.99 pg/ml by day 5 post-infection. In contrast, uninfected MRC-5 cells did not secrete detectable levels of IL-6 (Figure 3A). To determine the neutralizing activity of the anti-IL-6 IgY antibody, various concentrations of IgY (25, 50, 100, and 200 µg) were administered to virus-infected cells on day 3 post-infection. The results showed that secreted IL-6 levels significantly decreased in a dose-dependent manner when compared to untreated cells (0 µg). The inhibition percentages on day 4 post-infection were determined as 60.73±3.30, 82.20±1.40, 83.60± 0.75, and 92.53±0.77, respectively (Figure 4A). In addition, we examined whether the reduction in IL-6 levels after treatment affected overall virus production. The results indicated that anti-IL-6 IgY treatment had no effect on virus production (Figure 3B). Altogether, these findings demonstrate that anti-IL-6 IgY effectively neutralizes IL-6 produced during HCoV-OC43 infection.
Anti-IL6 IgY neutralize IL6 from LPS stimulated cell: In addition to viral infections, the LPS component found in many pathogenic bacteria can stimulate IL-6 production. In this study, MRC-5 cells were treated with 1 µg/mL LPS. This concentration of LPS induced IL-6 production, reaching a maximum concentration of approximately 200 pg/ml, whereas untreated cells did not secrete IL-6. Secreted IL-6 was detectable on day 1 and peaked on day 2 following LPS stimulation (Figure 5). As expected, treatment with various concentrations of anti-IL-6 IgY resulted in nearly complete neutralization of secreted IL-6 in the supernatant at 1 and 2 days post-treatment (Figure 6).
Discussion :
This study demonstrated the production of polyclonal anti-IL-6 antibodies from the egg yolk of recombinant IL-6 immunized chickens. The resulting IgY antibodies were specific, produced in high yield, and exhibited active neutralizing properties. These findings highlight an efficient platform for generating anti-IL-6 polyclonal IgY antibodies using laying hens. Notably, specific antibodies were rapidly detectable in egg yolk shortly after immunization and persisted in hens for over 90 days. This result aligns with the study by Woolley and colleagues 26, which reported that specific antibodies appeared in eggs by day 6 after primary immunization, indicating that IL-6 is a potent immunogen. In this study, IgY was extracted from egg yolks using the ammonium sulfate precipitation method, yielding an average of 11.88 mg per egg. However, the purity of the obtained IgY was relatively low, averaging 66.11% and varying among samples. To enhance purity, alternative purification methods showing high purity could be implemented, such as the sequential addition of 2% caprylic acid followed by ultrafiltration 27 or employing additional steps such as hydrophobic chromatography and sequential anion exchange 28. In this study, the specificity of the anti-IL6 IgY antibody was evaluated using ELISA and Western blot against recombinant IL-6 (rIL-6). However, we did not specifically assess cross-reactivity with other pro-inflammatory cytokines or IL-6 family members, such as IL-11, IL-27, IL-31, IL-35, oncostatin M, and leukemia inhibitory factor 29. Determining cross-reactivity is crucial for future studies to ensure the antibody’s specificity and to rule out potential off-target interactions, which could impact its reliability in diagnostic or therapeutic applications For in vitro models to assess the antibody's capability to neutralize IL-6, IgY with a high antibody titer (approximately 4.0 as determined by ELISA) and a purity of over 80% was selected to ensure that anti-IL-6 IgY was the major component and that activity originated from the specific anti-IL6 IgY.
Although FDA-approved therapeutic monoclonal antibodies such as Tocilizumab target IL-6R, antibodies directly targeting IL-6 are also of interest. Because IL-6, released from cells in response to stimuli, activates cells through both classical signaling (binding to IL-6R on the cell membrane) and trans-signaling (binding to sIL-6R) pathways, blocking IL-6 directly could be a promising strategy. Furthermore, monoclonal antibody production is complex and costly, making the polyclonal antibody production described in this study as a practical and cost-effective alternative for therapeutic and diagnostic applications. However, the potential limitations of IgY should also be considered, particularly in chronic or repeated administration in humans. One key concern is the potential for immunogenicity, as prolonged use of IgY could lead to the production of anti-IgY antibodies, which may reduce therapeutic efficacy. However, IgY does not activate the human complement system, and the risk of severe immune reactions is lower than with mammalian IgG. Additionally, polyclonal antibodies recognize multiple epitopes on an antigen, whereas monoclonal antibodies target a single epitope. In some cases, the presence of diverse antibody species in a polyclonal preparation could lead to epitope competition or steric effects, potentially influencing antigen binding or functional activity. However, these effects depend on factors such as antibody concentration, epitope accessibility, and antigen structure. These aspects are important considerations for future studies, particularly in the development of IgY-based therapeutics.
IL-6 and other cytokines are elevated in patients with pathogen infections, particularly severe COVID-19, with IL-6 playing a key role as a predictor in the hyperinflammatory state 30. Treatments targeting IL-6 or its receptor (IL-6R) have been associated with clinical improvement and reduced mortality 31. In this study, we characterized anti-IL6 IgY to evaluate its ability to bind IL6 released from cells in response to stimuli in an in vitro setting.
HCoV-OC43-infected cells was used as a model in this study. HCoV-OC43, one of the seven known human coronaviruses, is responsible for causing the common cold. This virus infects human lung fibroblast MRC5 cells, triggering IL-6 production 32. Thus, the HCoV-OC43 model provides a safe model for studying coronaviruses, including SARS-CoV-2.
Findings of the present study showed that treatment with anti-IL6 IgY neutralized IL-6 secreted from HCoV-OC43-infected MRC5 cells in a dose-dependent manner. After treatment, IL-6 levels drastically decreased, indicating the direct neutralizing effect of anti-IL6 IgY on soluble IL-6. However, IL-6 levels began to rise again two days after the treatment. Importantly, anti-IL6 IgY did not directly affect the virus itself, suggesting that the resurgence of IL-6 levels post-treatment was driven by ongoing viral activity. These results support those treatments targeting IL-6 or IL-6R in patients with severe coronavirus infections primarily help by mitigating the hyperinflammatory state rather than directly suppressing viral replication.
Dysregulation of the inflammatory system during sepsis is potentially lethal and is mainly caused by bacterial infection. IL-6 is an important marker for sepsis. Under normal conditions, the concentration of IL-6 in serum is less than 5 pg/ml. During sepsis, the level of IL-6 increases rapidly and remains consistently elevated, often exceeding 500 pg/ml 33,34. Blockade of IL-6 or IL-6R using specific inhibitors can mitigate the incidence of sepsis and reduce mortality 35. In this study, we demonstrated that 50 µg/ml of anti-IL-6 IgY efficiently inhibited IL-6 release from LPS-treated MRC-5 cells which released a high level of IL-6. Further investigation into the effect of anti-IL-6 IgY binding to IL6 secreted from LPS-treated MRC-5 cells may be required to explore its application as an alternative treatment for high IL-6 associated conditions.
Conclusion :
Our findings highlight that anti-IL-6 IgY, produced from laying hens, specifically targets IL-6 and efficiently inhibits it in vitro models. This indicates the potential of anti-IL-6 IgY as an alternative treatment to alleviate cytokine storm syndrome and cytokine release syndrome in patients suffering from pathogen infections and systemic inflammatory response syndrome.
Acknowledgement :
This research was supported by Faculty of Veterinary Science, Mahidol University, Thailand (MUVS0003/2565) for animal husbandry and testing. The animal experiment was approved from Faculty of Veterinary Sciences, Mahidol University-Institute of Animal Care and Use Committee (IACUC COA No. MUVS 2022-02-07). The recombinant protein production and antibody characterization were supported by the Institute of Molecular Biosciences, Mahidol University. The antibody purification was supported by Faculty of Tropical Medicine, Mahidol University. The authors would like to thank Mr. Somnuek Palabodeewat for his technical support with in vitro testing.
Conflict of Interest :
The authors declare no conflict of interest.
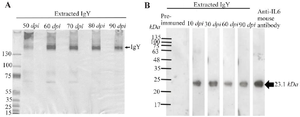
Figure 1. Purity and specificity of extracted anti-IL-6 IgY. A) Representative extracted samples were analyzed by SDS-PAGE under non-reducing conditions to determine the purity of the extracts. B) Western blot analysis of anti-IL-6 IgY extracted from egg yolks at the indicated days post-immunization (dpi) in chickens.
|
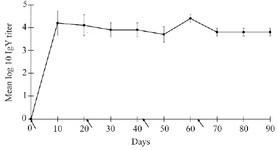
Figure 2. Average anti-IL-6 IgY titer from three independent experiments were determined by in-house ELISA. Arrows represent the day of immunization.
|

Figure 3. IL-6 production and HCoV-OC43 titer in virus-infected MRC-5 cells. A) IL-6 production from MRC-5 cells infected with HCoV-OC43 at an MOI of 0.1. B) HCoV-OC43 virus at the indicated days before and after treatment with anti-IL-6 IgY at concentrations of 0, 25, 50, 100, and 200 µg/ml.
|

Figure 4. Neutralizing activity of anti-IL-6 IgY in vitro using HCoV-OC43-infected cells. A) Levels of secreted IL-6 from MRC-5 cells infected with HCoV-OC43 at an MOI of 0.1, before and after treatment with anti-IL-6 IgY. Various concentrations of anti-IL-6 IgY were infected to virus-infected cells on day 3 post-infection, and IL-6 levels in the supernatant were determined by ELISA. Asterisks indicate statistical significance at p<0.05. B) Inhibition of IL-6 production by HCoV-OC43-infected MRC-5 cells after incubated with anti-IL-6 IgY.
|
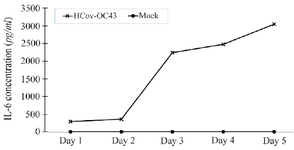
Figure 5. IL-6 production from MRC-5 cells after LPS stimulation.
|

Figure 6. Neutralizing activity of anti-IL-6 IgY in vitro in LPS-stimulated cells. A) Levels of secreted IL-6 from LPS-stimulated MRC-5 cells before and after treatment with anti-IL-6 IgY. Various concentrations of anti-IL-6 IgY were added, and IL-6 levels in the supernatant were determined by ELISA. Asterisks indicate statistical significance at p<0.05. B) Inhibition of IL-6 production by LPS-stimulated MRC-5 cells on day 4 post-stimulation after incubated with anti-human IL-6 IgY.
|
|