Effect of Intra-ovarian Injection of Mesenchymal Stem Cells or its Conditioned Media on Repeated OPU-IVEP Outcomes in Jersey Heifers and Its Relationship with Follicular Fluid Inflammatory Markers
-
Sarvari, Ali
-
Department of Theriogenology, Faculty of Veterinary Medicine, University of Tehran, Tehran, Iran
-
Reproductive Biotechnology Research Center, Avicenna Research Institute, ACECR, Tehran, Iran
-
 Niasari-Naslaji, Amir
Department of Theriogenology, Faculty of Veterinary Medicine, University of Tehran, Tehran, Iran, Tel: +98 21 61117100, 22432020; Fax: +98 21 6693322, 22432021; E-mail: niasari@ut.ac.ir
Niasari-Naslaji, Amir
Department of Theriogenology, Faculty of Veterinary Medicine, University of Tehran, Tehran, Iran, Tel: +98 21 61117100, 22432020; Fax: +98 21 6693322, 22432021; E-mail: niasari@ut.ac.ir
-
Department of Theriogenology, Faculty of Veterinary Medicine, University of Tehran, Tehran, Iran
-
 Shirazi, Abolfazl
Reproductive Biotechnology Research Center, Avicenna Research Institute, ACECR, Tehran, Iran, Tel: +98 21 61117100, 22432020; Fax: +98 21 6693322, 22432021; E-mail: shiraziabbas@yahoo.com, a.shirazi@avicenna.ac.ir
Shirazi, Abolfazl
Reproductive Biotechnology Research Center, Avicenna Research Institute, ACECR, Tehran, Iran, Tel: +98 21 61117100, 22432020; Fax: +98 21 6693322, 22432021; E-mail: shiraziabbas@yahoo.com, a.shirazi@avicenna.ac.ir
-
Heidari, Banafsheh
-
Department of Photo Healing and Regeneration, Medical Laser Research Center, Yara Institute, ACECR, Tehran, Iran
-
Borjian Boroujeni, Sara
-
Reproductive Biotechnology Research Center, Avicenna Research Institute, ACECR, Tehran, Iran
-
Moradi, Mohammad Hossein
-
Department of Animal Sciences, Faculty of Agriculture and Natural Resources, Arak University, Arak, Iran
-
Naderi, Mohammad Mehdi
-
Reproductive Biotechnology Research Center, Avicenna Research Institute, ACECR, Tehran, Iran
-
Behzadi, Bahareh
-
Reproductive Biotechnology Research Center, Avicenna Research Institute, ACECR, Tehran, Iran
-
Dehghan, Mohammad Mehdi
-
Department of Surgery and Radiology, Faculty of Veterinary Medicine, University of Tehran, Tehran, Iran
Abstract: Background: Repeated Ovum Pick Up (OPU) could have a detrimental effect on ovarian function, reducing In Vitro Embryo Production (IVEP). The present study examined the therapeutic effect of adipose–derived Mesenchymal Stem Cells (MSCs) or its Conditioned Medium (ConM) on ovarian trauma following repeated OPU. Resolvin E1 (RvE1) and Interleukin-12 (IL-12) were investigated as biomarkers.
Methods: Jersey heifers (n=8) experienced 11 OPU sessions including 5 pre-treatment and 6 treatment sessions. Heifers received intra-ovarian administration of MSCs or ConM (right ovary) and Dulbecco’s Modified Phosphate Buffer Saline (DMPBS; left ovary) after OPU in sessions 5 and 8 and 2 weeks after session 11. The concentrations of RvE1 and IL-12 in follicular fluid was evaluated on sessions 1, 5, 6, 9, and 4 weeks after session 11. Following each OPU session, the IVEP parameters were recorded.
Results: Intra-ovarian administration of MSCs, ConM, and DMPBS did not affect IVEP parameters (p>0.05). The concentration of IL-12 in follicular fluid increased at the last session of pre-treatment (Session 5; p<0.05) and remained elevated throughout the treatment period. There was no correlation between IL-12 and IVEP parameters (p>0.05). However, RvE1 remained relatively high during the pre-treatment and decreased toward the end of treatment period (p<0.05). This in turn was associated with decline in some IVEP parameters (p<0.05).
Conclusion: Intra-ovarian administration of MSCs or ConM during repeated OPU did not enhance IVEP outcomes in Bos taurus heifers. The positive association between RvE1 and some of IVEP parameters could nominate RvE1 as a promising biomarker to predict IVEP parameters following repeated OPU.
Introduction :
In the dairy industry, embryo production technologies including in vivo (Multiple Ovulation and Embryo Transfer-MOET) and In vitro Embryo Production (IVEP) through ultrasound-guided transvaginal Ovum Pick Up (OPU) could speed up the genetic gain by more than 30% per year 1-5. Any attempts to promote OPU-IVEP outputs could assist the widespread use and cost-effectiveness of this technology in dairy cattle and buffalo industries. Unfortunately, long-term OPU in cattle and buffalo could decline IVEP 6,7. Repeated OPU sessions may lead to severe problems initiated by inflammation and terminated by stromal fibrosis, adhesions, and thickening of the tunica albuginea 8,9. It could also increase plasma FSH/LH concentration, change follicle growth rate and co-dominancy 8,10, which in turn could increase the risk of cystic ovarian disease 11.
Both the oocyte and the embryo are very susceptible to alteration in their micro-environments 12,13. Serum metabolic changes are reflected in the follicular fluid 14. Consequently, any changes in the composition of follicular fluid might have an impact on oocyte and cumulus cell quality 15,16. This in turn could affect embryo metabolism and may alter the gene expression patterns, embryonic development, and implantation, leading to developmental abnormalities 17-20. Acute inflammation is an immediate response to tissue damage 21. Alterations in the follicular fluid content following acute or chronic inflammation could affect oocyte quality and embryo development 22-24. Therefore, it seems that any approach to reduce ovarian trauma following repeated OPU could improve IVEP outcomes.
A considerable body of information is available on the therapeutic effects of stem cells on ovarian diseases and hypofunctions. Mesenchymal Stem Cells (MSCs) derived from bone marrow 25,26, adipose tissue 27,28, menstrual blood, and umbilical cord 29 were used to treat ovarian-induced dysfunction in animal models. Although the possibility of MSCs conversion to oocytes is not clear, the paracrine role of intra-ovarian administration of MSCs to treat ovarian dysfunction is more likely evident 30. Adipose-derived MSCs have adaptability and anti-inflammatory properties and strong immunomodulatory characteristics to modulate a variety of immune cells both in vitro and in vivo. They can escape the immune recognition systems and alter the host's defense mechanisms 31-34, and act as a promising candidate for treating diseases in both human and animal studies 35. The regenerative properties of MSCs within their micro-environment could be due to the modulation of inflammatory processes and the release of different bioactive molecules including certain cytokines and growth factors 31-36, angiogenic 37, anti-apoptotic 25,38, and anti-fibrosis 39-41 factors, and the enhancement of folliculogenesis through improvements of blood perfusion 26,41,42 and neovascularization leading to the repair of the injured ovary 43,44. Therefore, it is hypothesized that an MSC-conditioned medium could contain a variety of cytokines and extracellular vesicles that could regulate the immune response 45,46 and appear to provide therapeutic benefits for ovarian injury 26,28,47.
The therapeutic effect of stem cells differs among various species 48. Compared to studies conducted in laboratory animals, there are very few publications on stem cell therapy in large animals, particularly in cattle. In the initial study in Bos indicus cows, Soares et al revealed that ovarian injection of MSCs enhanced the OPU-IVEP efficiency 49. Recently, a similar beneficial impact of adipose-derived MSCs on OPU-IVEP was reported following artificially induced acute ovarian damage in Bos indicus cows 7. To the best of our knowledge, there is no study on the therapeutic effect of intraovarian MSCs administration on Bos taurus cattle following repeated OPU.
Exploring biomarkers to suggest ovarian damage could be a valuable tool to predict OPU-IVEP outcomes in cattle. In this context, cytokines could be potential biomarker candidates. These proteins can influence cell differentiation and cell chemotaxis, stimulate or inhibit cell proliferation, modulate the expression of other cytokines 50, assist in regulating the ovarian cycle 51, promote follicular development, steroidogenesis, activate and recruit leukocytes required for ovulation, as well as tissue remodeling during ovulation, luteinization, and luteolysis 50. Among cytokines, Interleukin-12 (IL-12) was considered a cytotoxic substance at high concentrations. Therefore, excessive amounts of IL-12 in the follicular fluid could affect the natural process of folliculogenesis, oocyte quality, ovulation, and implantation 52-54. Another potential group of biomarkers is Resolvins. They are lipid mediators derived from omega-3 polyunsaturated fatty acids that act on a local inflammatory milieu to prevent leukocyte recruitment and stimulate repairment. Resolvin E1 (RvE1) is produced from omega-3 eicosapentaenoic acid and exhibits powerful anti-inflammation/pro-resolution effects in vivo 55,56. RvE1 increases oocyte quality in vitro by reducing cumulus cell apoptosis and increasing cell survival and proliferation 57.
The aim of the present study was to administer adipose-derived MSCs or its conditioned medium into the ovary, as two therapeutic approaches, in order to alleviate inflammation in Bos taurus (Jersey) heifers experiencing repeated OPU. We evaluated oocyte and embryo development parameters and also measured IL-12 and RvE1, as two potential follicular fluid biomarkers, to correlate with embryo production in OPU-IVEP programs.
Materials and Methods :
Unless otherwise stated, all reagents were purchased from Sigma-Aldrich Chemical Co (St. Louis, MO, USA), and plastic wares were purchased from Corning Inc. (Corning, NY, USA). All experiments were performed according to the relevant ethical guidelines and regulations.
Donors: The present study was performed in the Biofarm Center of Avicenna Research Institute, Alborz Province, Iran. Healthy cyclic Jersey heifers (n=8), 12 to 14 months of age, were selected as donors and received a total balanced mixed ration with free access to water.
Experimental design: Before starting the main experiment, heifers (n=8) had four OPU sessions, at 7-day intervals (Pre-experiment period; Figure 1). No data was recorded during the pre-experimental period. OPU sessions: 1 to 3, with 7-day intervals, and OPU sessions: 3 to 5, with 14-day intervals, were considered as control (Pre-treatment sessions; Figure 1). During OPU sessions: 6 to 11 (Treatment sessions, Figure 1), the same heifers were assigned into two groups. The right ovary received either intra-ovarian injection of adipose-derived mesenchymal stem cells in DMPBS (MSCs; n=4) or conditioned medium retrieved from MSCs culture medium (ConM; n=4; Figure 1). In both treated groups, the left ovary was considered as control and received Dulbecco’s Modified Phosphate Buffer Saline (DMPBS). OPU sessions 6 to 8 and 9 to 11 were conducted at 2 and 4-week intervals, respectively (Figure 1). The number of follicles (observed and aspirated) with different diameters (large, medium, and small follicles), the number of oocytes [recovered, Germinal Vesicle (GV), MII, denuded, cultured oocytes] and the number of embryos were recorded in all OPU sessions. All recovered Cumulus-Oocyte Complexes (COCs) except the degenerated COCs and MII oocytes were cultured. The follicular fluids of the left and right ovaries of all animals were collected separately following OPU sessions: 1, 5, 6, 9, and four weeks after session 11 in order to measure IL-12 and RvE1 (Figure 1).
Isolation and culture of mesenchymal stem cells (MSCs): Adipose tissue was obtained from the base of the tail of one Jersey heifer. After meticulously cleaning the base of the tail, epidural anesthesia was administered using lidocaine hydrochloride 2% (Vetacaine®, Aburaihan Co., Iran) and xylazine (Xyla®; Interchemie, Holland). A 3-5 cm incision at the base of the tail was made to remove approximately 5 g of adipose tissue. This tissue was thoroughly cleaned in a cold saline solution that was supplemented with streptomycin (400 µg/ml; S1277) and penicillin (400 IU/ml; P3032). The tissue was then minced with a sterile scalpel blade and digested for 30 min with 0.001% type I collagenase (Gibco BRL) diluted in alpha Minimum Essential Medium (α-MEM; Gibco BRL) with streptomycin (100 µg/ml; S1277), penicillin (100 IU/ml; P3032). After digestion for 3 hr at 38.5°C, collagenase activity was neutralized by the addition of an equal volume of α-MEM containing 15% Fetal Bovine Serum (FBS; Gibco BRL). The treated tissue was centrifuged at 300 g for 10 min, and the pellet was re-suspended in α -MEM supplemented with 15% FBS. A second centrifugation step was performed at 300 g for 10 min, and the pellet was re-suspended and cultured on a 75 ml flask, with the same culture medium. The culture medium was changed 24 hr after cell culture and then every 48 hr. Cells cultured in α-MEM with 15% FBS were trypsinized when they reached 75-80% confluency. The cells were cultured with a density of 7×105 cells in each 75 ml flask and cultured for two passages.
Quality control test of MSCs: The quality of cells was validated using the guidelines of the International Conference on Harmonization Q2 (ICH Q2). There was no fungal and/or bacterial contamination, determined using direct and indirect Mycoplasma tests, and endotoxin in the culture medium.
In vitro differentiation: Before the intraovarian administration of MSCs, their stemness potency was characterized by their abilities to differentiate into osteogenic, adipogenic, and chondrogenic cells in commercial differentiation media (Stem Cell Technology Research Center, SCTRC, Iran). For osteogenic differentiation, 3×104 cells at the second passage were cultured on culture dishes overnight. The culture medium was then replaced with a commercial osteocyte differentiation medium (SCTRC, Iran) containing DMEM, FBS, Dexamethasone, β- Glycerol Phosphate, and Ascorbic Acid. The cells were cultured in this medium for three weeks.
For adipogenic differentiation, cells were cultured in a commercial adipocyte differentiation medium (SCTRC, Iran) containing DMEM, FBS, Indomethacin, IBMX (3-isobutyl-1-methylxanthine), insulin, and dexamethasone for three weeks as previously described.
For chondrogenic differentiation, cells were plated as described above and cultured for 3 weeks in a chondrocyte differentiation medium (TERMRG- ARI, Iran) containing a-MEM, insulin, TGF-ß1, and ascorbic acid-2-phosphate. For all 3 differentiation protocols, the culture medium was changed and the cells were monitored for morphological changes every third day.
Differentiation analysis: To confirm cell differentiation, the cells were washed twice with DMPBS, fixed with 4% paraformaldehyde in DMPBS for 15 min at room temperature, and washed again in DMPBS and stained. Briefly, for osteogenic differentiation, calcium deposition was visualized by staining cells with 2% Alizarin Red S in water, pH=4.1, as previously reported 58 (Figure 2). For chondrogenic differentiation, the presence of proteoglycans in the cells was verified by staining the cells with 1% Alcian Blue in 3% acetic acid, pH=2.5 59. Stained cells were visualized under an inverted microscope at 400X magnification (Nikon Eclipse 50i, Nikon Instruments Inc.). For adipogenic differentiation, intracellular accumulation of lipid-enriched vacuoles was visualized by staining the cells with 1.25% Oil Red O in 30% isopropanol for 10 min at room temperature, followed by washing with 60% isopropanol 59,60 (Figure 2).
Condition medium (ConM) preparation: MSCs cells (the second passage) were seeded (2.8× 106 cells in T75 cm2 flasks) in DMEM supplemented with 10% FBS medium until reaching 80 to 90% confluency. It was rinsed three times with DMPBS and then for another 48 hr in serum-free DMEM containing penicillin and streptomycin and cultured in an atmosphere containing 5% CO2 at 37°C. After 48 hr, the culture medium was collected and centrifuged for 5 min at 1200 g to remove cell debris. The supernatant was sterilized by filtration through a 0.2 µm syringe filter. Prepared ConM were aliquoted and stored at -80°C until the day of the experiment.
Intra-ovarian administration of MSCs, ConM, and DMPBS: The frozen MSCs were thawed and cultured until 75-80% confluency. After trypsinization and neutralization, harvested cells (2.5×106) were washed twice with DMPBS. The cell suspension in DMPBS (700 µl) was loaded into a 1 ml syringe. Under ultrasound scanning, MSCs suspension (700 μl; Right ovary) was divided into three equal fractions and injected into three regions of the ovarian cortex, free from antral follicles or luteal tissue using an 18 G needle. A similar injection procedure was applied for ConM (700 µl; Right ovary) and DMPBS (700 µl; left ovary).
Ovarian stimulation: Two days after ablating dominant follicles, animals received FSH (Cinnal-f®, CinnaGen pharmaceutical company, Iran), every 12 hr, in decreasing doses (110, 70, 60, 60 IU). OPU was performed 36-48 hr after the last FSH injection.
Ovum Pick Up (OPU): Before follicle aspiration, the animals received epidural anesthesia with lidocaine hydrochloride 2% (Vetacaine®; Aburaihan Co. Iran) and xylazine (Xyla®; Interchemie, Holland). The perineal region was thoroughly cleaned and disinfected with alcohol and betadine. All follicles larger than 3 mm were aspirated by a disposable 18 G biopsy needle using an ultrasound scanner (iuStar-160 vet; United Imaging Healthcare, China) equipped with a micro convex probe (R11 MCA, 4-9 MHz). A vacuum pressure of 50 to 80 mmHg was applied for aspiration of the follicles. The follicular fluid of the left and right ovary was collected in two separate 50 ml tubes containing 10 ml of aspiration solution (DMPBS with 2% FCS and 5 IU/ml sodium heparin (Caspian Co., Iran), for measuring OPU- IVEP parameters. In sessions 1, 5, 6, 9, and four weeks after session 11, in which RvE1 and IL-12 concentration were measured the aspiration solution volume was decreased to the constant volume of 0.5 ml to prevent more dilution of follicular fluid. The dilution was considered for the calculation of real concentrations of the target substances. Prepared follicular fluids were stored at -80°C until the day of IL-12 and RvE1 measurement.
In Vitro Maturation (IVM): IVM medium consisted of TCM199 (M5017) supplemented with 0.1 IU/ml FSH (F8174), 0.01 IU/ml LH (L5269), 1 IU/ml 17 β estradiol (E2257), 0.1 mM Cysteamine (M9768), 0.3 mg/ml L-Carnitine (A6707), 100 ng/ml IGF (I8779), 10 ng/ml EGF (E9644), and 10% FCS. Oocytes were cultured in a humidified incubator with 5% CO2 at 38.5°C for 24 hr.
In Vitro Fertilization (IVF): After IVM, expanded COCs were washed and transferred to In Vitro Fertilization (IVF) medium consisting of Tyrode's albumin lactate pyruvate supplemented with 10 µg/ml heparin, 20 µM D-penicillamine, 10 µM hypotaurine, and 1 µM epinephrine. All COCs were incubated with the same type of sexed semen of Jersey Bull of proven fertility at a density of 1×106 spermatozoa/ml. IVF lasted for 20 hr in a humidified incubator with 5% CO2 at 38.5°C.
In Vitro Culture (IVC): The cumulus cells and sperms were removed from presumptive zygotes after vortexing for 1 min in the HEPES-SOF medium. The presumptive zygotes were cultured in drops of IVC medium consisting of synthetic oviductal fluid consisting of supplemented with essential (MEM; 20 µl/ml, M7145) and non-essential amino acids (BME; 10 µl/ml, B6766) under mineral oil. On Days 3-8, IVC medium was supplemented with 2.5% charcoal-stripped serum, 2.5% Platelet-Rich Plasma (PRP), and 0.15 mg/ml L-Carnitine (A6707). Four µl of Culture Medium (CM) per embryo (5 embryo/20 µl) was considered during culture. During IVC, the zygotes were cultured in a humidified atmosphere with 5% CO2, and 5% O2 at 39°C until days 7 and 8. The in vitro-produced embryos were evaluated on Day 3 to detect cleaved zygotes and on Days 6 to 8 to detect morula and blastocyst.
Assessment of resolvin E1 and interleukin-12: The concentration of RvE1 was assayed by bovine highly sensitive RvE1 ELISA kit (Cat. No: ZB-hs12042C-Bo9648; ZellBio GmbH, Germany), with the assay range of 2.5-80 pg/ml and sensitivity of 0.3 pg/ml. The concentration of IL-12 was assayed by a bovine Interleukin-12 ELISA kit (Cat. No: ZB-10211C-Bo9648; ZellBio GmbH, Germany), with the assay range of 7.5-240 ng/L and sensitivity of 0.9 ng/L.
Statistical analysis: Data were analyzed using Proc Mixed of SAS (version 9.1, SAS Institute, Cary, NC, USA). An individual cow was used as the experimental unit. Initially, the Shapiro-Wilk test was used to evaluate the normality. The repeated measure was applied for analyzing the ovarian sites and treatment effects, with OPU sessions as the repeated variable. The interaction between ovarian sites (left or right) and OPU sessions was analyzed during the pre-treatment period (sessions 1-5) and since the effect of ovarian sites was not significant, the interaction between treatments, including DMPBS (administered into the left ovary), MSCs (administered into the right ovary) and ConM (administered into the right ovary), and OPU sessions were analyzed during OPU sessions 6 to 11.
The following statistical equation was used for quantitative traits: Yijk=µ+αi+βj+αβ(ij)+c(αi)+ε(ij)k, in which Yijk is dependent variable; µ is the grand mean, αi is the effect of the i-th treatment (or ovary); βj was the effect of time; αβij is the interaction effect between treatment (or ovary) and time; c(αi) was the random effect of cows within the treatment, and ε(ij) was the overall error term. Discrete data were analyzed using the Proc Genmod procedure with either binomial or poisson distributions included in the model. Least Square Means (LSMs) were obtained using the Tukey Post Hoc test and differences were considered significant if they were less than 0.05. Data were presented as mean±SEM.
Results :
During the pre-treatment period (Sessions: 1 to 5), there was no interaction between sessions and ovaries (p>0.05; Tables 1-3) in any parameters of OPU and IVEP. During the treatment period (Sessions: 6-11), there was no interaction between sessions and treatments in any parameters of OPU and IVEP (p>0.05; Tables 1-3). During the pre-treatment period (Sessions: 1-5), there was no difference between the right and left ovaries in OPU and IVEP parameters (p>0.05; Tables 1-3). There was also no difference between treated (right ovary) and untreated (left) ovaries in OPU and IVEP parameters during the treatment period (Sessions: 6 to 11; p>0.05; Tables 1-3). Therefore, data for experimental groups were pooled together and 16 ovaries per session were considered to analyze the effect of treatment throughout sessions (Figures 3-6). The number of the largest follicles decreased from session 1 to 3 and remained constant afterward (Figure 3). The number of small and medium size follicles did not follow a particular pattern and displayed fluctuations over time (Figure 3). Similar patterns were noticed in the number of oocytes, zygotes, cleavage and morula embryos during sessions 1 to 5 (p<0.05) but not during sessions 6 to 11 (p>0.05; Figures 4 and 5). Number of total blastocysts decreased significantly from session 7 to 11 compared with the session 6 (p<0.05; Figure 6). There was no interaction between sessions and ovaries during the pre-treatment period and between sessions and treatments during the treatment period in IL-12 and RvE1 (p>0.05; Table 4). There was no difference in the concentration of IL-12 and RvE1 in the follicular fluid of the left and right ovaries (p>0.05; Table 4). Therefore, data for experimental groups were pooled together and the trends over sessions were investigated (Figure 6).
During the pre-treatment period (Sessions: 1 to 5), IL-12 increased significantly from the first to the last session (p<0.05), but RvE1 concentration did not change significantly in the same time frame (p>0.05; Figure 6). During the treatment period (Sessions: 6 to 11), the concentration of IL-12 did not change until the end of the experiment (Session 11; p>0.05; Figure 6); however, RvE1 decreased during the treatment period (Session: 6 to 11; p<0.05; Figure 6). There was no correlation between IL-12 concentration and any of OPU and IVEP parameters throughout the experiment (Sessions: 1 to 11; p>0.05; Table 5). There were significant correlations between RvE1 and large (r=0.24; p<0.05) and medium size (r=0.24; p<0.05) follicles, the cleaved zygote (r=0.46; p<0.01), morula (r=0.46; p<0.01) and blastocyst (r=0.29; p<0.01) during the treatment period (Table 5).
Discussion :
The main purpose of the present study was to alleviate the possible destructive impact of the repeated OPU on IVEP results using intra-ovarian administration of MSCs or its conditioned medium in Bos taurus (Jersey) heifers. By progressing OPU sessions during the treatment period, the number of oocytes, zygotes, cleaved embryos, and morula remained constant but blastocyst rates decreased significantly. This confirms the previous findings in Bos indicus cows 7 and buffalo 6. There are discrepancies among reports regarding the effect of repeated OPU on the number of aspirated follicles and retrieved oocytes. Repeated OPU could enhance the number of retrieved oocytes but not their quality 58. This observation is not supported by Hasler, who observed a significant decrease in the number of oocytes as OPU sessions proceeded 59. On the other hand, Kruip et al found high repeatability in the number of oocytes retrieved over several sessions 60. In other studies, there was no significant difference among OPU sessions over a period of eight weeks in follicle numbers 61, and collected oocytes 62.
This study did not show any benefit of intra-ovarian administration of MSCs or its conditioned medium on OPU-IVEP parameters. Most studies on the therapeutic effects of stem cells in ovarian dysfunction have been conducted on mice and rats and to a lesser extent on other animals as well as in humans for the treatment of ovarian dysfunction and endometrial abnormalities 43,63. MSCs are multipotent stem cells that are widely used as a therapeutic approach compared to other stem cells due to a lack of ethical concerns in their use, safety, no immune rejection, and easier and more accessible sampling with less damage 64. Previous studies, mainly in pigs, have reported the beneficial effects of adipose-derived MSCs on wound healing 65. It has been shown that intraovarian injection of MSCs in rats has a therapeutic effect on damaged ovaries caused by cyclophosphamide (an anticancer drug) 66 and Premature Ovarian Insufficiency (POI) caused by tripterygium glycosides 67. Results of the present study did not show the healing effect of adipose-derived MSCs injected into the ovaries of Jersey heifers (Bos taurus) and did not confirm the previous result in Nellore (Bos Indicus) cows in which a positive effect of intra-ovarian injection on OPU-IVEP outcomes was reported 7. According to previous studies, possible hypotheses for the observed discrepancy may be related to factors such as the necessity for activation of MSCs, cell origin, number of injected cells, time of administration, nature, and age of damage or inflammation, the immunomodulatory capacity of MSCs, and different cattle breed. It seems that additional manipulation of the ovary after cell therapy, similar to what happened in our study, performing OPU after cell therapy, in contrast to previous studies on rats and mice, is one of the most important reasons for this discrepancy. Since microenvironmental factors have a significant role in the activation and performance of immunomodulatory functions of MSCs 68-70, the in vitro culture and proliferation of MSCs may strongly change their phenotypic, differentiation, and immunomodulatory characteristics 71-72. Moreover, inflammation appears to increase the expression of immune-related genes in MSCs 73,74. In other words, these cells are not natural modulators of the immune system and must be activated by pro-inflammatory cytokines after being injected into the ovary to show their immune-modulatory properties 33,74-77. In addition, the complexity and specific function of the damaged tissue environment may cause different therapeutic effects of MSCs 69. Acute injury, which is characterized by the production of IL-1, IL-6, or TNF-α, has activated MSCs compared to chronic lesions that are characterized by the activation of T cells and/or IFN-γ. The injected MSCs are activated by the local inflammatory environment within the affected area 74. Therefore, it is important to know the acute or chronic nature of the inflammatory site into which MSCs are injected. The difference in breeds of cows might also express the different inflammatory state of the ovary following OPU which could partially explain the difference in the results of the present study in Bos taurus heifers and the previous study in Bos indicus cows 7. Moreover, Bos indicus dairy breeds produced a higher number of COCs (Gyr: 23.8) 78; Nellore: 29.6) 7 per OPU than Bos taurus dairy breeds [(Holstein: 19.3) 78; Jersey: 10.9, current study]. The greater number of follicles aspirated following OPU, the greater area of damage produced within the ovary. Additionally, in the previous study in Bos indicus cows 7, the investigators applied 30 additional punctures with a 16 G needle to induce more inflammation in order to induce acute ovarian injury. The strategy of inducing acute inflammation might be considered as a trigger for MSCs to be activated and produce a useful environment for healing trauma induced by OPU. The lower level of inflammation that occurred during routine OPU, similar to the current study, might not be a sufficient stimulus to activate and reveal the therapeutic properties of MSCs. Furthermore, if our goal is to reduce ovarian damage following repeated OPU, it seems unreasonable to induce more ovarian damage by additional ovarian puncture as performed in the previous study 7. The lack of therapeutic effect of MSCs on chronic ovarian inflammation in the study of Malard et al calls for further investigation of the behavior of bioactive molecules in the niche of ovarian inflammation 7. In this regard, the measurement of follicular fluid biomarkers to determine the state of ovarian inflammation that leads to the activation of MSCs seems to be a critical prerequisite for the treatment of damaged ovaries with MSCs. Therefore, the measurement of biomolecules such as IFN-γ and TNF in follicular fluid as a marker that leads to the activation of MSCs seems to be reasonable 79-81. Another strategy could be to activate MSCs in vitro which may have benefits to treat damaged ovaries. Previously, it has shown that activated T cells alone or in combination with cytokines such as interferon-g (IFN-g) or tumor necrosis factor (TNF-α), as well as signals through Toll-like receptors (TLR3 and TLR4), enhance the potential role of MSCs in modulating the immune response in vitro 70,76,82.
The use of MSCs’ conditioned medium in the present study did not enhance OPU-IVEP outcomes. This finding is against the therapeutic effect of MSCs’ conditioned medium on inflammatory arthritis 83, damaged cartilage 84, porcine IVF 85, and enhancing ovarian function in Polycystic Ovary Syndrome (PCOS) and Premature Ovarian Failure (POF) patients 28. Regarding the use of stem cell‐conditioned media compared to stem cell, there are some advantages such as less production time and cost, no need for immunological compatibility between donor and recipient, less stimulation of immune reactions, the ability to use harmless strategies such as freeze‐drying, without the need for sensitive cell storage methods 47. Most bioactive molecules produced by MSCs are released through exosomes which have similar functions to MSCs in tissue repair and regeneration, but less is known about their immunomodulatory effect 86. Exposure of MSCs to pro-inflammatory stimuli such as IFN-, TNF-, IL-1, lipopolysaccharide (LPS), IL-4, or hypoxia activates the production of exosomes containing varying levels of bioactive peptides such as growth factors and anti-inflammatory agents 87,88. The growth factors subsequently stimulate the development of fibroblasts, endothelial cells, and tissue progenitor cells, which assist tissue regeneration and repair 36. Therefore, the failure in the repairing effect of MSCs’ conditioned medium on ovarian tissue in the present study could be due to the lack of exposure to biological stimulants that activate MSCs to produce therapeutic molecules. Hypothetically, in vitro activation of MSCs prior to collection of the conditioned medium could enhance the healing properties of such medium.
In the present study, the concentration of IL-12 in follicular fluid elevated in the fifth session of the pre-treatment period compared to the first one, and remained elevated throughout the treatment period, displaying possible trauma from the fifth OPU session onward. However, there was no correlation between IL-12 and other IVEP parameters. There is controversy about the effect of IL-12 on the development of oocytes and embryos. IL-12 is a pro-inflammatory cytokine and regulates cell-mediated immune responses 51. The p40 subunit of IL-12 is shared with IL-23 and is essential for recruitment and activation of many inflammatory cell types. Both of these cytokines interact with the innate and adaptive immune systems 89. Positive correlation was found between IL-12 and oocyte quality, fertilization, and embryo development in women; however, it suggested a dose-dependent role for IL-12 in the follicles 51,90. A majority of findings have indicated that IL-12, particularly at excessive levels, is negatively associated with folliculogenesis, oocyte quality, embryo quality, and implantation 52-54,91.
RvE1 remained relatively high during the pre-treatment period and then decreased during the treatment period from session 6 to 11 which was associated with the decline in some IVEP parameters. Interestingly, once RvE1 reached the concentration of 9.1 pg/ml in session 9, it was concurrent with the decrease in the number of produced blastocysts. This is consistent with human findings in which follicular fluid concentration of RvE1 below 8.96 pg/ml may be associated with poor oocyte quality 57. RvE1 is an aspirin-modified cyclooxygenase-2, which is produced locally from cumulus cells. RvE1 increases oocyte quality in vitro by reducing cumulus cell apoptosis and increasing cell survival and proliferation. Therefore, RvE1 could be used as a potential biomarker to predict suboptimal oocytes 57. However further studies with more animals in the experiment will empower the findings.
Conclusion :
In conclusion, the intra-ovarian administration of MSCs or its condition medium could not enhance IVEP outcomes following repeated OPU in Bos taurus heifers. The association between RvE1 and some of the IVEP parameters suggested RvE1 as a potential biomarker for the prediction of IVEP outcomes following repeated OPU. However, IL-12 was unable to provide significant association with IVEP parameters at the condition of this study.
Acknowledgement :
The authors would like to thank the Avicenna Research Institute (ARI-ACECR; Research Grant No. 990106-016), and the National Science Foundation of Iran (Research Grant No. 4000306) for their financial support. Biofarm Center is also acknowledged for providing laboratory and field facilities. This study was approved by the Ethics Committee of the Academic Center for Education, Culture and Research, Tehran, Iran (Code: ir.acecr.avicenna.rec.1400.009).
Conflict of Interest :
The authors have no conflicts of scientific interest with respect to the manuscript.
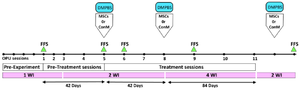
Figure 1. Experimental design to investigate the effect of intra-ovarian administration of MSCs or its conditioned medium following repeated OPU in Jersey heifers. The experiment was initiated following four pre-experimental OPU sessions (Pre-experiment). The main experiment includes 5 pre-treatment and 6 treatment sessions. During the treatment session intra-ovarian administration of MSCs or ConM (right ovary) and Dulbecco’s Modified Phosphate Buffer Saline (DMPBS; left ovary) was conducted after each OPU session. OPU: Ovum Pick UP; WI: Week Interval between two OPU sessions; FFS: Follicular Fluid Sampling; MSCs: Mesenchymal stem cells; ConM, MSCs’ Conditioned Medium; DMPBS: Dulbecco Modified Phosphate Buffer Saline.
|
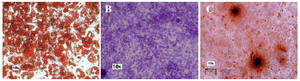
Figure 2. In vitro differentiation of mesenchymal stem cells derived from adipose tissue of Jersey heifer. Adipocyte (A); Chondrocyte (B); Osteocyte (C).
|
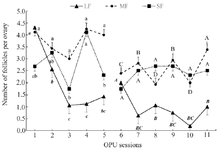
Figure 3. Average number of large (LF, >10 mm), medium (MF, 5-10 mm), and small follicles (SF, <5 mm) in ovaries of Jersey heifers (n=8) during 11 OPU sessions (16 ovaries were pooled and analyzed per session). Statistical analysis of data in pre-treatment (1 to 5) and treatment (6 to 11) sessions were analyzed separately. Data were presented as mean±SEM. ab) Values within the group during sessions 1-5 with different letters differ (p<0.05).
AB) Values within the group during sessions 6-11 with different letters differ (p<0.05).
|
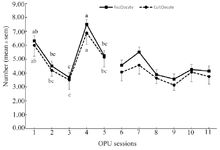
Figure 4. Number of recovered and cultured oocytes derived from left and right ovaries of Jersey heifers (n=8) during 11 OPU sessions. Statistical analysis of data in pre-treatment (1 to 5) and treatment (6 to 11) sessions were analyzed separately. Data were presented as mean±SEM.
ab) Values within the group during sessions 1-5 with different letters differ (p<0.05). There was no significant difference between consecutive values within the group during sessions 6-11 (p>0.05).
|
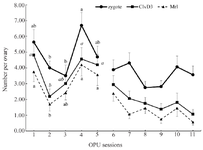
Figure 5. Number of Zygote, Day 3 cleaved (ClvD3) and Morula (Mrl) stage embryos of COCs derived from left and right ovaries of Jersey heifers (n=8) during 11 OPU sessions. Statistical analysis of data in pre-treatment (1 to 5) and treatment (6 to 11) sessions were analyzed separately. Data were presented as mean±SEM. Ab) Values within the group during sessions 1-5 with different letters differ (p<0.05). There was no significant difference between consecutive values within the group during sessions 6-11 (p>0.05).
|

Figure 6. The follicular fluid concentrations of Resolvin E1 (RVE1 pg/ml) and Interleukin-12 (IL-12 ng/L) in different OPU sessions (1, 5, 6 and 9) and 4 weeks after session 11 and the total number of blastocysts produced on Day 7 and Day 8 (TotD7D8) in Jersey heifers (n=8) that were subjected to 11 OPU sessions and received intra-ovarian administration of MSCs or ConM (right ovary) and Dulbecco’s Modified Phosphate Buffer Saline (DMPBS; left ovary) on OPU sessions 5 and 8 and 2 weeks after session 11. Statistical analysis of data in pre-treatment (1 to 5) and treatment (6 to 11) sessions were analyzed separately. Data were presented as mean±SEM. Ab) Values within the group during sessions 1-5 with different letters differ (p<0.05). AB) Values within the group during sessions 6-11 with different letters differ (p<0.05).
|
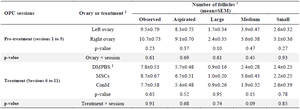
Table 1. Interaction between ovaries (left and right) and pretreatment/treatments sessions on the number of follicles in Jersey heifers
† Large follicle: >10 mm; Medium follicle: 5-10 mm; Small follicles: <5 mm.
‡ DMPBS, Dulbecco’s Modified Phosphate Buffer Saline administered into left ovary; MSCs, Mesenchymal stem cells administered into right ovary; ConM, MSCs’ conditioned medium administered into right ovary.
|
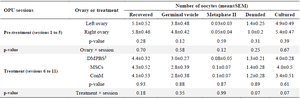
Table 2. Interaction between ovaries (left and right) and pretreatment/treatment sessions on the number of retrieved oocytes in Jersey heifers
‡ DMPBS, Dulbecco’s Modified Phosphate Buffer Saline administered into left ovary; MSCs, Mesenchymal stem cells administered into right ovary; ConM, MSCs’ conditioned medium administered into right ovary.
|
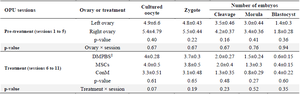
Table 3. Oocyte developmental competence following OPU. Data were presented per session as mean± SEM
‡ DMPBS, Dulbecco’s Modified Phosphate Buffer Saline administered into left ovary; MSCs, Mesenchymal stem cells administered into right ovary; ConM, MSCs’ conditioned medium administered into right ovary.
|
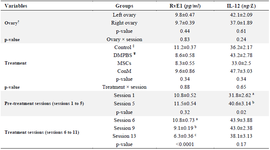
Table 4. The concentration of Interleukin-12 (IL-12) and Resolvine-E1 (RvE1) in follicular fluid of left and right ovaries, in pre-treatment (Sessions 1-5), and treatment sessions (Sessions 6-11). Data were presented as mean± SEM
abc: Values within column with different superscripts differ (p<0.05). Control (without injection); DMPBS (Dulbecco’s Modified Phosphate Buffer Saline) injected to left ovaries; MSCs (Mesenchymal stem cells) and ConM (MSCs’ Conditioned Medium) injected to right ovaries separately.
† Mean of RvE1 and IL-12 in FF sampling (sessions: 1,5,6,9,13) of right or left ovaries
‡ Control: Mean of RvE1 and IL-12 in FF sampling in sessions 1 and 5
¥ DMPBS, MSCs or ConM: Mean of RvE1 and IL-12 in FF sampling in sessions 6, 9 and 11.
|
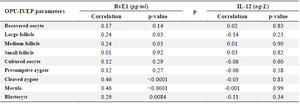
Table 5. Correlation of Resolvine-E1 (RvE1) and Interleukin-12 (IL-12) concentrations in follicular fluid with varying size of follicles, oocyte, and embryo development
|
|