Is Formulary of Maranta Arundinacea Clarias Gariepinus (F-MaCg) a Potential Immunostimulant?
-
 Fitri Jamil , Kurnia
Department of Internal Medicine, Faculty of Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia, Tel: +62651 7555591; E-mail: kurnia_jamil@unsyiah.ac.id
Fitri Jamil , Kurnia
Department of Internal Medicine, Faculty of Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia, Tel: +62651 7555591; E-mail: kurnia_jamil@unsyiah.ac.id
-
Darmawi, Darmawi
-
Department of Veterinary Medicine, Faculty of Veterinary Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia
-
Usman, Said
-
Department of Public Health, Faculty of Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia
Abstract: Background: External factors have the potential to act as immunostimulants in order to influence the body's protection from many foreign antigens. We intended to investigate the ethanol extract Formulary of F-MaCg effect as an immunostimulant.
Methods: A purely experimental with a completely randomized design was used on twenty-four white male rats. They were divided into four groups:1) G0 [given aquades (5 ml)]; 2) G1 [given F-MaCg-75 mg/gr BW (Body Weight)]; 3) G2 (F-MaCg -150 mg/gr plus Hepatitis B vaccine at the beginning and the end of treatment); and 4) G3 (F-MaCg -300 mg/gr BW plus hepatitis B vaccine at the end of treatment). The rat's spleen lymphocyte blast transformation was evaluated on the 15th and 37th days. Lymphocytes were examined using microtetrazolium assays. Optical Density (OD) was measured using an ELISA reader [493 nμ (nanomicro)]. Observation of lymphocyte viability by a counting chamber using a light microscope and trypan blue 1 % before being cultured with Phytohaemoaglutinin.
Results: Lymphocyte cell viability in the hepatitis B vaccine-induced group on the 15th day showed the highest average value in the G2 (1,484/mcl of blood); on the 37th day, it was in G3 (1,578/mcl of blood). The proliferative activity of spleen lymphocytes indicated by the difference in the OD values of the four treatment groups was 0.467, 0.913, 1.619, and 1.473 nμ, respectively. Histological observations of the spleen showed differences at all given formulary dose concentrations.
Conclusion: F-MaCg could be an immunostimulant because of its ability to trigger a cellular immune response.
Introduction :
The arrowroot tuber’s crop is rich in vitamins and minerals that contain most of the essential micronutrients of the human body. The tuberous rhizomes are rich in starch (type 2 resistant starch) and have various chemical components such as alkaloids, carbohydrates, cardiac glycosides, proteins, amino acids, phenolic compounds, terpenoids, saponins, flavonoid, and gums 1-3. Type 2 resistant starch, flavonoid compounds, and saponins can modulate specific immune responses to increase the production of cytokines as mediators of immune responses and induce humoral responses, thereby improving antibody production 4. Saponins also reduce the digestibility of proteins; this is made possible by forming saponin complexes-easily digestible proteins 5.
Likewise, the nutrients found in dumbo catfish have high mineral content and low saturated fatty acids 6. In addition, dumbo catfish has a superior protein quality compared to meat. This protein is a nutrient that is needed in the formation of enzymes for the body’s metabolism, including the immune system 7. Lack of protein levels has an impact on decrease in the immune system, making it easier for infectious diseases to attack the body 8,9.
Various studies have been conducted on extracts of arrowroot bulbs and African catfish separately 8-11. However, concerning the cellular immune response, the information is minimal, especially the materials used in the form of a formulary (combination). Based on these data findings, it needs more in-depth tests to know the effect of F-MaCg as an immunostimulant and new adjuvant in the white rat. Henceforth, we evaluated the cellular immune response, including viability and spleen lymphocyte blast transformation in rat-induced hepatitis B vaccine at the beginning and the end of treatment.
Materials and Methods :
This study used a purely experimental with a Completely Randomized Design (CRD) with four treatments and six repetitions to analyze the effects of the F-MaCg formulary as immunostimulants in rats. The F-MaCg formulary is an ethanolic extract from the combination of arrowroot tuber (Maranta arundinacea) and dumbo catfish (Clarias gariepinus).
All experimental procedures were reviewed and approved by the Institutional Review Board, which approved the study by the Veterinary Ethics Committee of the Faculty of Veterinary Medicine, Syiah Kuala University, Banda Aceh, Indonesia, certificate number: 146/KEPH/VI/2022.
Experimental Subjects: The researcher used white male rats (Rattus norvegicus) (n=24) because they have many anatomical functions similar to humans and quickly respond to the treatment given. They were divided into four groups consisting of:
1. Negative control group (C-) or G0: given aquades water (5 ml)
2. Positive control group (C+) or G1: given F-MaCg-75 mg/gr BW (Body Weight)
3. G2: given F-MaCg-150 mg/gr + Hepatitis B vaccine induction at the beginning of treatment + Hepatitis B vaccine booster*.
4. G3: given F-MaCg-300 mg/gr BW + Hepatitis B vaccine at the end of treatment*.
*Booster and the end of treatment vaccines were given 30 days after the vaccine at the beginning of the treatment.
Basic F-MaCg formulary of dose determination: This study used a dose of 150 mg/gr-BW/day as an empirical dose (n). This study used three doses by increasing and decreasing the empirical dose based on the arithmetic progression formula: 1/2n, n, 2n. The dosage of the formulary used in this study:
Dosage I : ½ n = ½×150=75 mg/gr BW/day
Dose II : n = 150 mg/gr BW/day
Dose III : 2 n = 2×50=300 mg/gr BW/day
The induction dose of the Hepatitis B vaccine: The usage of the Hepatitis B vaccine in this research is to see whether the administration of the F-MaCg formulary can modulate increasing the vaccine's immune response. Furthermore, we chose the Hepatitis B vaccine to stimulate the immune system because there are still responsive and unresponsive cases when administering the vaccine. The basis for determining the dose for experimental animals refers to the conversion table of human to animal dose. The standard dose is 1 ml in adult humans. Adult human dose conversion factor (70 kg) to rat (200 g) based on the conversion table is 0.018. Adult human dose × conversion dose to the rat was 200 gr.
1 ml×0.018 = 0.018 ml
The induction dose given to white rats was 0.018 ml.
CRBC (cow red blood cells) production process: 1. 20 ml of blood was taken from the jugular vein in the cattle's neck and was put into a centrifugation tube and centrifuged at 1500 rpm speed for 15 min to separate it from the plasma.
2. We discarded the supernatant, then washed it several times with Bovine Phosphate Serum (BPS) pH=7.4.
3. In addition, we obtained 100% CRBC suspension after the washing process was completed.
4. BPS added 100% CRBC suspension with the same volume to obtain 50% CRBC.
5. We took 0.2 ml of 50% CRBC and added 10 ml of BPS to obtain 1% CRBC levels.
Treatment of test animals and induction of 1% CRBC: The induction of rats with CRBC aims to increase binding with antibodies because CRBC has a more substantial negative charge. All test animals were orally treated with test extract preparations according to the administration volume for seven days except group G0. Immunostimulant activation tests for G1, G2, and G3 groups induced 1% (1 ml) CRBC antigen intraperitoneally on the third day. Furthermore, they were reinduced 1% v/v (0.1 ml) CRBC on the seventh day intraplantar. Test animals' leg volume was measured on the seventh day before and after induction of 1% CRBC antigen intraplantar at 4, 24, and 48 hr using a digital plethysmometer.
Spleen lymphocyte blast transformation test: The rat spleen lymphocyte blast transformation test was carried out 15 days after the first vaccination and seven days after the final vaccination (37th day). Lymphocytes were cultured on microplate 24 with a 1×106 cells/ml density and examined using MTT (microtetrazolium) assays. Optical Density (OD) was measured using an ELISA reader at a wavelength of 493 nm. Observation of lymphocyte viability using a counting chamber with the help of a light microscope with the addition of trypan blue 1% before being cultured with PHA (Phytohaemoaglutinin). In this observation, there was no residue after culture with PHA.
The method used to make ethanol extract from arrowroot tubers: Arrowroot starch extraction was carried out by referring to the method developed by Lingga 12 with modifications to optimize the manufacture of arrowroot starch. Starch was made through peeling, washing, soaking, drying, milling, and sifting, using the following steps:
1. After all the ingredients are cleaned, put them in a grinder, add enough water, and grind until smooth.
2. The milled material was squeezed out with a batiste (filter) and repeated up to three times with the addition of sufficient water. The juice is collected and deposited for 24 hr.
3. Furthermore, precipitated starch was slowly separated from the water by the suction technique, and the starch was dried in an oven at 50°C for 1 hr.
4. The dried starch was ground using a grinder and sifted with mess no 20 (starch-1 or primary extraction).
5. The arrowroot flour extraction used the maceration method (water and 40% ethanol); furthermore, the extract was concentrated by vacuum to obtain dry extract using a vacuum dray.
6. The starch was suspended in an HCl solution with a solution ratio of acid: starch 1:1 (w/v) and hydrolyzed
for 6 hr in a shaking incubator at 35°C.
7. The starch solution is immediately neutralized with NaOH until it reaches a pH of 6, then centrifuged with a rotational speed of 3300 rpm so that it can separate residue from the supernatant. The starch residue was washed several times with distilled water to remove traces of minerals.
8. The acid-hydrolyzed starch is dried in a drying oven at 50°C and sifted through a mess sieve no 60 (starch-2 or final product) because the acid hydrolysis process affects the increase in the amylose content of arrowroot starch and will affect the increase in levels of dietary fiber and resistant starch.
The explanation of arrowroot starch extraction is shown in flow stage (Figure 1).
The method used to make ethanol extract from African catfish: Making African catfish extract used a defined processing flow: weeding, washing, slicing, milling, and weighing, using the following steps:
1. We separated the meat from the head, tail, spines, fins, skin, and entrails. Clean the fish and cut the meat carefully so as not to stick to the bones and remove the head.
2. To sterilize fish from adhering impurities such as metal, the water used for washing and rinsing was treated with an ozonizer using Agroculture Treatment Machine (ATM) for 15 min (for 20 L of water). It can help to kill harmful bacteria such as Escherichia coli (E. coli), Salmonella, Vibrio, and Colli-form, then input the phytase enzyme to increase the nutritional value.
3. We prepared a pot of stew and boiled the water.
4. After the water was boiled for about 5 min, we added the fish. The function of boiling is essential because fishmeal will increase protein and fiber levels and minimize ammonia and ash content.
5. We let it stand for 12 hr, then we drained and squeezed the fish with a press until the fish oil separated from the meat.
6. We carried out the anaerobic fermentation for 24 hr. Fermentation functions to remove methane gas and sterility, then dried in an oven for 1 hr at a temperature of 50°C and continued in the grinder and sieve with mess no.60. The explanation of African catfish starch extraction is shown in flow stage (Figure 2).
Arrowroot bulbs and African catfish ratio in F-MaCg formulary: Arrowroot bulbs and African catfish ratio in F-MaCg Formulary is shown in table 1.
Results :
Data from table 2 showed that all treatment groups performed changes before and after antigen (CRBC 1%) induction. It was characterized by the presence of an inflammatory process in the experimental animals' legs. There was no change in the normal group (without any treatment). In the 3 test groups given 75 mg/gr BW, 150 mg/gr BW, and 300 mg/gr BW, increased swelling of the legs volume, especially in T-4, T-24, and T-48, was observed after CRBC 1% antigen induction.
Based on figure 3, the highest viability of lymphocyte cells on day 15 found in G3 was equal to 1.528+1.48 microcentiliter (mcl)/blood, followed by G2 1.428+1.40 mcl/blood, G1 1.154+1.17 mcl/blood and the lowest viability found in G0 0.110+0.17 mcl/blood. The highest viability of lymphocyte cells on day 37 found in G3 was equal to 1.672+1.578 mcl/blood, followed by G2 1.445+1.433 mcl/blood, G1 1.158+1.151 mcl/blood, and the lowest viability found in G0 0.110+0.182 mcl/blood.
Based on the results of the blast transformation test in the four treatment groups, the OD mean value which describes the reaction of lymphocyte proliferation of the spleen was obtained from each group (Figure 4). ANOVA test was performed to determine the difference in OD values from 4 treatment groups. The results showed a difference between the OD mean values in the four treatment groups. Furthermore, the DMRT test was carried out and showed that there was a difference between G1 and G2 group, and there was a difference between the G1 group and G3 group. The results of the MDRT test in groups that were vaccinated both at the beginning of treatment (group G2) and at the end of treatment (group G3) proved that there was a difference between the G2 group and the G3 group.
Spleen histological examinations was performed to see the appearance of the white pulp, marginal center, and spleen weight (Figure 5). White pulp size, spleen weight, and germinal center showed differences in each treatment group with different formulary dose concentrations. The negative control group (G0) showed no different changes in the diameter of the white pulp, germinal center, and spleen weight because of not being treated (F-MaCg). Meanwhile, in the G1 (75 mg F-MaCg dose), G2 (150 mg F-MaCg dose), and G3 (300 mg F-MaCg dose), there was a change in the diameter of the white pulp, germinal center, and spleen weight (Table 3), and there was no decrease in the rat spleen weight. At a concentration of 300 mg of F-MaCg, necrotic cells previously performed in G1 and G2 were not found.
Discussion :
Based on observations, previous studies have only focused on one of the extracts, namely arrowroot tuber extract or catfish extract. For example, studies that had used arrowroot extract showed significant increase in IgG, IgM, and IgA serum levels 8; provided a mucosal immune response, and improved the physical properties of feces 13; inhibited E. coli bacteria as a prebiotic to yogurt 14; and reduced MDA, SGPT, and SGOT 15.
One of the studies which used catfish extract was a study on the effect of catfish flour substitution on the acceptability and nutritional value of gluten and casein-free cookies in children with Autism Spectrum Disorder (ASD) who have digestive disorders. The results show that gluten and casein-free cookies are very feasible as a high-calcium and good protein snack as an alternative snack for children with ASD 16.
The viability test in the vaccinated group showed lymphocyte values greater than in the unvaccinated group. Furthermore, re-vaccination (booster) in G2 increased the viability and proliferation of lymphocytes. The swelling was found in T-4, T-24, and T-48 due to the activation of T cells that stimulate the release of cytokines (IL-12) in producing IFN-ɣ by increasing the cytolytic function of Natural Killer (NK) cells and T cells that stimulate the secretion of IFN-ɣ which will activate macrophages to eliminate antigens 17-19. The process of increasing activation of macrophages in producing cytokines causes the accumulation of cytokines (IL-12) at the site of induction, indicating a hypersensitivity reaction of a slow type.
According to the theory of slow-type hypersensitivity (type IV), the increase in leg volume occurs within 6-12 hr and reaches its maximum intensity after 24-72 hr. In T-24 and T-48 began a decrease in the leg volume of the white rat (Rattus norvegicus). However, a decline was more dominant in the T-48. This showed that the administration of F-MaCg formulary affects the acceleration of the immune response so that the process of reducing the swelling occurred more quickly. Thus, administration of F-MaCg formulary had the ability to act as an immunomodulator. The presence of flavonoid compounds in arrowroot tuber plants, carboxylic compounds, and amino acid content in catfish made these two materials potentially as an immunomodulator 9,14-17.
Increased proliferation of spleen lymphocyte cells in vaccinated white rats indicated that stimulated lymphocytes will cause biochemical changes accompanied by cell division. In this study, lymphocytes were stimulated by PHA, which acts as a mitogen and then lymphocytes will carry out the proliferation process. In the G2 group, revaccination (booster) had a great impact on increasing the viability and proliferation of lymphocytes. In the group of vaccinated white rats at the end of treatment, a marked increase in the proliferative ability of lymphocytes was obsreved. It is indicated by the activation and differentiation of lymphocytes.
There was a difference in lymphocyte proliferation response between the group of vaccinated white rats and unvaccinated white rats (group G1). Calculation of viability showed a difference between the three treatment groups. In this study increasing number of lymphocyte viability was not followed by an increase in lymphocyte proliferation, thus the ability of lymphocyte proliferation in white rats is not only driven by PHA but also the presence of external factors (F-MaCg). Blast transformation describes lymphocytes that have been stimulated by F-MaCg and then will carry out the process of dividing themselves. Stimulated lymphocytes will undergo biochemical and morphological changes. Biochemically, there was a change in the speed of oxidative metabolism, protein, and RNA synthesis. Morphologically, the transformation of the blasts is characterized by an increase in the diameter of the cells, the chromatin becomes loose and pale daubed and within 8-12 hr these changes can be observed with the help of a light microscope as a lymphoblasts.
Thus, blast transformation can be used as a marker that lymphocytes are stimulated by mitogen or antigen and other materials which are capable of triggering a cellular response 2. Lymphocytes are cells that play a role in the immune response because they have the ability to recognize antigens through special surface receptors and divide into a number of cells with identical specificity and a long life span making them ideal cells for adaptive responses. Two large populations of lymphocytes are T and B lymphocytes. Pathogenic B-cells and T-cells will develop when B cells and T cells are activated by memory. Throughout the life span, the memory cells will "remember" any particular pathogen encountered and may respond strongly if the pathogen is detected again 20,21. A repeat vaccination (booster) is an additional vaccination after administration of a previous dose and serves as a booster or repeat dose intended to boost immunity to that antigen to protective levels after a specified period 3,22-24.
There was a change in the diameter of the white pulp, germinal center, and spleen weight. It happened because the F-MaCg formulary has a concentration-dependent bimodal effect in stimulating and suppressing the immune system and is thought to be related to the activation of the NF-kB gene, which encodes the formation of interleukins and TNF-α. In addition, it can stimulate the immune system by increasing phagocytic activity and increasing the number of leukocytes. The white pulp and the germinal center are the gathering places for lymphocytes, especially T lymphocytes. Meanwhile, the B lymphocyte cells gather in the marginal zone of the white pulp.
The diameter change of the white pulp and germinal center indicates immune system enhancement 25. The white pulp diameter change is possible due to the flavonoid compounds in the formulary. Flavonoids as immunomodulators impact the increase of white pulp diameter, lymphocytes, and splenocytes proliferation index. Several other studies also explain that herbarium containing flavonoid elements can increase immune cells in the spleen.
Spleen weight is one of the parameters used to measure the level of toxicity of a given formulary material. The spleen weight loss indicates the cellular activity decrease in damaged or dead spleen tissue. A spleen weight is generally 0.2% of body weight or 0.72-1.10 g 26,27. There was no decrease in the weight of the rat spleen when treated with concentrations of 75, 150, and 300 mg mg of F-MaCg. At 300 mg of F-MaCg concentration, some necrotic cells previously performed in G1 and G2 were not found. Thus, the administration of F-MaCg at all dose concentrations did not show symptoms of spleen toxicity in the form of atrophy of the spleen 27,28.
Conclusion :
Administration of ethanolic extract formulary of arrowroot tubers and catfish (F-MaCg) can trigger a cellular immune response. It is marked by increasing viability and lymphocyte proliferation activity in the spleen of white rats induced with the hepatitis B vaccine. Activation of spleen lymphocyte cell proliferation was observed through the blast transformation test after being stimulated with PHA. Thus, it can be concluded that the administration of ethanolic extract formulary of arrowroot tubers and catfish flour can increase lymphocyte viability, and the administration of repeat vaccine can increase spleen lymphocyte proliferation.
Acknowledgement :
Our thanks go to Prof. Dr. Nurdin, Msi as the head of the organic laboratory at Syiah Kuala University in Banda Aceh; Prof. Ummu Balqis, Msi as a professor in the field of immunology, who has helped a lot in this research; Dr.dr. Zinatul Hayati, Sp.MK (K) as the Head of the Microbiology Laboratory of RSU. Zainal Abidin Banda Aceh; and Dr. Vivian Nanny Lia Dewi, M.Kes, a colleague who has helped a lot to finish writing this journal.
Conflict of Interest :
The authors declared no potential conflicts of interest, authorship, and publication of this article.
Financial support: this research article and publication is from the researcher's budget.
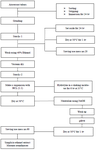
Figure 1. The method used to make ethanol extract from arrowroot tubers
|
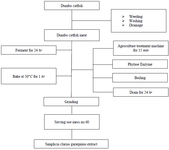
Figure 2. The method used to make ethanol extract from African catfish.
|
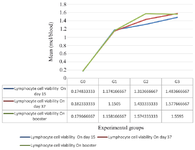
Figure 3. Lymphocyte cell viabilities (statistically analyzed with ANOVA and duncan).
|
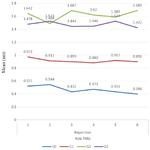
Figure 4. Results of OD Mean value on spleen lymphocyte prolife-ration in white rats induced with hepatitis B Vaccine (statistically analyzed with ANOVA and duncan)
|
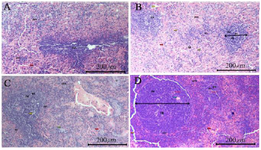
Figure 5. Differences in spleen histology, A) Spleen histology in the negative control group (G0), B) Spleen histology in the F-MaCg 75 mg/g Body Weight Group (G1), C) Spleen histology in the F-MaCg 150 mg/g Body Weight group (G2), D) Spleen histology in the F-MaCg 300 mg/g Body Weight group (G3).
Description: pm = Red pulp, pp = White pulp, pg = Germinal center, zm = Malignancy zone, as = Central artery, tr = trabeculae.
= Macrophage
= Blood components
= hydropic degeneration
= Plasma cell
|

Table 1. Formulary proportion and dosage mg/gr BW (Body Weight) of experimental animals
Description: A: Marantha arundinace starch, B: Clarias gariepinus starch, C-: Negative control group, C+: Positive control group, HBV: Hepatitis B Vaccine
|
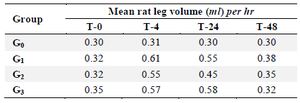
Table 2. Measurement result of white rats leg volume after CRBC 1% (cow’s red blood cells) induction
Description: F-MaCg: Formulary ethanolic extract of arrowroot tuber and dumbo catfish, CRBC: Cow red blood cells, T0: Initial measurement, T-4: Measurement at the 4th hr after antigen induction, T-24: Measurement at the 24th hr after antigen induction, T-48: Measurement at the 48th hr after antigen induction.
|
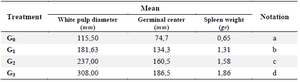
Table 3. Mean of white pulp diameter, germinal center and Spleen weight
a: Spleen histology in the negative control group (G0).
b: Spleen histology in the F-MaCg 75 mg/g Body Weight group (G1).
c: Spleen histology in the F-MaCg 150 mg/g Body Weight group (G2).
d: Spleen histology in the F-MaCg 300 mg/g Body Weight group (G3).
|
|