Evaluation of PLGA-Encapsulated Recombinant GroEL of S. typhi immune Responses Against Enterohaemorrhagic and Enteropathogenic Escherichia coli
-
Parvane , Milad
-
Biology Research Center, Faculty of Basic Sciences, Imam Hossein University, Tehran, Iran
-
 Nazarian, Shahram
Biology Research Center, Faculty of Basic Sciences, Imam Hossein University, Tehran, Iran, E-mail: nazarian56@gmail.com
Nazarian, Shahram
Biology Research Center, Faculty of Basic Sciences, Imam Hossein University, Tehran, Iran, E-mail: nazarian56@gmail.com
-
Kordbacheh, Emad
-
Biology Research Center, Faculty of Basic Sciences, Imam Hossein University, Tehran, Iran
-
Fathi, Javad
-
Department of Bacteriology and Virology, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran
-
Student Research Committee, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran
-
 Minae , Mohamad Ebrahim
Biology Research Center, Faculty of Basic Sciences, Imam Hossein University, Tehran, Iran, E-mail: mminaii@ihu.ac.ir
Minae , Mohamad Ebrahim
Biology Research Center, Faculty of Basic Sciences, Imam Hossein University, Tehran, Iran, E-mail: mminaii@ihu.ac.ir
Abstract: Background: Heat Shock Proteins (HSPs) elicit humoral and cellular immune responses. Due to their high sequence homology, they can be developed as a new immunogen for cross prophylactic and vaccination effects against infectious agents such as Enteropathogenic and Enterohemorrhagic Escherichia coli (EPEC and EHEC). This study aimed to evaluate the immunogenicity and cross-protective efficacy of rGroEL of Salmonella typhi (S. typhi) encapsulated in poly lactic-co-glycolic acid (PLGA) nanoparticles against EPEC and EHEC.
Methods: Recombinant GroEL was expressed in Escherichia coli (E. coli) and purified using Ni-NTA affinity chromatography. The protein was encapsulated in PLGA by the double emulsion method, and the nanoparticles were characterized physicochemically. BALB/c mice were immunized, and the efficacy of the protein to elicit immune responses was assessed.
Results: Over-expression in E. coli led to corresponding 64.5 kDa protein bands in Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis (SDS-PAGE). Non-ag-gregated nanoparticles had a spherical shape with a mean diameter of 194.3±3 nm and encapsulation efficiency of 89.5±2.5%. Antibody isotyping revealed that GroEL immunization induced both IgG1 and IgG2a antibodies. Moreover, immunization of the mice with recombinant GroEL protein conferred 80 and 60% protection against lethal infections by EPEC and EHEC, respectively. Furthermore, organ burden studies revealed a significant reduction in infection in the immunized mice compared to the non-immunized ones. Passive immunization with anti-GroEL sera also protected 50% of the mice against the lethal doses of EHEC and EPEC strains.
Conclusion: The findings indicated that immunization of the mice with recombinant GroEL of S. typhi elicited cross-protection against other bacterial infections. This represented the immense potential of GroEL to be developed as a single vaccine against multiple pathogens
Introduction :
The prevalence of diarrheal diseases is one of the significant global health problems. Lack of preventive strategies such as vaccines, makes these diseases more prevalent 1-3. Diarrheal diseases are among the most common childhood sicknesses, with 750 thousand annual deaths in children under five years 4,5. Escherichia coli (E. coli) is one of the leading causes of diarrheal with several pathotypes. In addition to diarrheal and intestinal diseases, it can cause different gastrointestinal problems, including bacteremia, nosocomial pneumonia, and neonatal meningitis. One of these pathotypes is Enteropathogenic E. coli (EPEC), which colonizes the small intestine in infants and causes symptoms including diarrhea with fever, nausea, and vomiting. This pathotype is mainly endemic to developing countries and causes the disease in travelers and infants 1,6,7. The primary mechanism of EPEC pathogenesis is forming Attaching and Effacing (A/E) lesions, which cause attachment to the intestinal epithelium in the first stage, followed by the invasion of the bacteria into the intestinal tissues 8,9. Another subtype is Enterohemorrhagic E. coli (EHEC), whose pathogenesis is in the large intestine. EHEC causes abdominal pain, watery diarrhea, and bloody diarrhea, eventually leading to hemolytic uremic syndrome 10-12.
Due to the increasing antibiotic resistance and the unknown cellular and molecular processes during E. coli infection, developing effective vaccines using dominant immunogenic antigens is one of the most important strategies to combat this issue. Several studies and vaccine trials have been performed to prevent diarrheagenic E. coli 4,13. On the other hand, Heat Shock Proteins (HSPs) are conserved proteins involved in cellular processes such as protein folding, transport and can be induced under stressful conditions. They are considered a candidate for prophylactic and therapeutic vaccines against infectious agents, due to the structure of conserved HSPs and their ability to induce humoral as well as cell-mediated immune responses 14.
The GroEL has been used as an immunogen in several candidate vaccines. In a previous study, the mice vaccinated with the Bacillus Calmette-Guerin (BCG) vaccine responded with GroEL-specific CD8+ T cell immunity against Mycobacterium tuberculosis (M. tuberculosis) 15. Moreover, GroEL orthologs were shown to be immunogenic in diseased hosts, including animals infected with Helicobacter pylori 16, Ehrlichia risticii 17, Ehrlichia chaffeensis 18, and Pasteurella haemolytica 19. The results of these studies confirmed that a protective immunogen with the ability to elicit an immune response across species could be considered as a candidate vaccine against multiple pathogens 20. HSPs have a high homology amongst different bacterial species, and numerous studies have confirmed the in vitro and in vivo cross-protective efficacy of rGroEL of Salmonella typhimurium (S. typhimurium) against various bacterial pathogens. When they are administered on in vivo surfaces, native antigens may become a weak immunogen in the host immune response due to partial processing, inadequate absorption, and degradation 21. To solve this problem, the antigen can be encapsulated in nanoparticles. Poly Lactic-co-Glycolic Acid (PLGA) is a proper choice and can protect antigens under various adverse conditions. PLGA nanoparticles improves the immunogenicity of antigens by boosting their absorbance by the lymphoid tissue and controlling their release 22,23.
Considering what was mentioned earlier, the present study aims to evaluate the immunogenicity and protective efficacy of the rGroEL of Salmonella typhi (S. typhi) encapsulated in PLGA nanoparticles to investigate its protective effects against infection by multiple pathogens such as EPEC and ETEC.
Materials and Methods :
Bacterial strains, plasmids, and media: The bacterial strains used in this study were Enteropathogenic E. coli, enterohemorrhagic E. coli, S. typhi, and E. coli Rosetta strains. The pET28a plasmid was obtained from Novagen (USA). Luria-Bertani (LB) broth and LB agar, which were used to cultivate the bacterial strains, were obtained from Merck (Germany).
Bacterial culture and purification of DNA: The bacterial cells were cultured in the LB medium at 37°C. The recombinant strains were also cultured in LB broth/LB agar supplemented with 100 μg/ml kanamycin. Plasmid and genomic DNA molecules were purified using gene elute genomic DNA isolation kit and gene elute plasmid DNA isolation kit (Sigma) according to the manual of the manufacturing company.
Cloning of grol gene: The DNA sequence of grol gene was obtained from the Gene Bank (Accession number: AAA85277.1). The S. typhi strain was cultured in LB broth, and the bacterial genome was extracted via the CTAB-NaCl method. The grol gene from S. typhi was amplified through Polymerase Chain Reaction (PCR) using the following sets of primers:
Forward primer: 5′-CGC GGA TCC ATG GCA GCT AAA GAC GTA AAA TTC-3′
Reverse primer: 5′-CGC AAG CTT TTA CAT CAT GCC GCC CAT ACC-3′.
PCR was performed under the following conditions: 94°C for five min (initial denaturation), 94°C for 40 s (denaturation), 65°C for 40 s (annealing), and 72°C for one min and 20 s (extension). A total of 35 PCR cycles were carried out to provide the final amplified product. The primer sequences were specifically composed to provide the final products containing BamH1 and HindIII restriction sites at 5′ and 3′ ends respectively. To purify the PCR products, the PCR purification kit (Bioneer Daejeon, Korea) was applied. pET28a vector and PCR products were digested with BamH1 and HindIII restriction enzymes and were purified before proceeding with the ligation reaction. Ligation was performed with T4 DNA ligase at 14°C for an overnight, and the mixture was transformed into fresh prepared competent E. coli BL21 (DE3) cells. Colony PCR and restriction digestion analysis were used to confirm the transformed colonies. DNA sequencing of the recombinant plasmid were performed for confirmation of cloning grol gene into pET28a expression vector.
Expression of recombinant GroEL protein: In order to produce the recombinant GroEL protein, the E. coli Rosetta strain received the recombinant pET28a-grol vector. To select the transformed colonies, LB agar plates were used. After selecting the recombinant colonies, they were cultured in 2×YT broth media containing the same antibiotics until they reached 0.7 OD at 600 nm. In order to induce protein expression, 1 mM Isopropyl-b-D-thiogalactopyranoside (IPTG) was added to the media at 37°C. Then, protein expression was evaluated at 2, 3, 4, and 16 hr. Upon centrifugation, the resultant bacterial pellets were gathered to be stored at –20°C prior to protein purification. Expression of the recombinant protein of interest was confirmed through Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) in a gel containing 12% (W/V) polyacrylamide (SDS gel apparatus; BioRad) pursued by Coomassie dyeing using blue color.
Purification of recombinant GroEL via Ni-NTA column: Since the final protein products were fused with the His-tag sequence, GroEL recombinant products were purified with native conditions using a Nickel Nitrilotriacetic Acid (Ni-NTA)-agarose column in accordance with the Qiagen manual of the product. In this step, the bacterial pellets were dissolved in the buffer of lysis A (20 mM Tris/HCl, 200 mM NaCl, 10 mM Imidazole, pH=8) and were put on ice for 60 min. Next, they were lysed by sonication for 20 min. The supernatant was retrieved upon cell lysate centrifugation at 4000×g at 4°C for 20 min. The product obtained in this step was loaded on the Ni-NTA purification column (Qiagen). After three times washing with buffer B (20 mM Tris/HCl, 200 mM NaCl, 20 mM Imidazole, pH=8), the target proteins were eluted using 250 mM imidazole elution buffer (20 mM Tris/HCl, 200 mM NaCl, 250 mM imidazole). The purified GroEL protein was further analyzed on 12% SDS-PAGE. The purified proteins were dialyzed against PBS1×. In addition, the final concentrations were estimated using the Bradford protein method and spectrophotometric analysis via the Nanodrop device. The final products were stored at -70°C.
Verification of recombinant proteins using western blotting: The recombinant protein was evaluated by Western blotting. The recombinant protein was electrophoresed on 12% SDS-PAGE gel and transformed onto nitrocellulose paper using a transfer buffer (20 mM Tris-base, 120 mM glycine, and 20% methanol). Nitrocellulose paper was blocked with the blocking buffer (PBST: 4.3 mM Na2HPO4 + 0.05% Tween, 137 mM NaCl, 2.7 mM KCl with 5% skim milk) for 12 hr at 4°C and then washed with PBST. Anti-His-tag HRP-conjugated antibody (ab18184, Abcam, Cambridge, MA, USA) was prepared 1/10000 in PBST buffer, added to nitrocellulose paper, and incubated for 1 hr. The membrane was washed three times with PBST and 3, 3´-diamino-benzidine reagent (DAB, Sigma-Aldrich, St. Louis, MO, USA). As chromogenic substrate was added reaction was stopped by washing the membrane with distilled water.
Preparation of PLGA (polylactide-co-glycolide) nanoparticles encapsulated with the recombinant protein: The protein was encapsulated in the PLGA (Sigma) polymer at the 50 PLA:50 PGA ratio (Mw 40000-75000) using a water/oil/water double emulsion (w/o/w) method. Then, 25 mg of PLGA was dissolved in 2 ml of dichloromethane and 1 ml of an aqueous protein solution containing 1 mg of the recombinant protein. Emulsification process was performed on ice by an ultrasonicator at the amplitude of 5 for 20 s. The w/o primary emulsion was added to 10 ml of 2% (w/v) aqueous Poly Vinyl Alcohol (PVA) in a drop-wise manner, which was then emulsified with a high-speed homogenizer at 12000×g for 2 min. The w/o/w double emulsion was added to 0.5% PVA and was stirred to allow the solvent to evaporate. After that, nanoparticles were collected, washed with sterile deionized water, and freeze-dried using 2.5% trehalose dihydrate as the cryoprotectant. Encapsulation efficiency was determined by hydrolyzing the nanoparticles. In doing so, 0.5 mg of the prepared nanoparticles were dissolved in 0.5 ml of 1 M sodium hydroxide and incubated at 37°C for 14 hr. The solution was neutralized by adding 0.5 ml of 1 M hydrochloric acid. After all, the solution was centrifuged, the supernatant was analyzed for protein content, and the Encapsulation Efficiency (EE) was calculated 24.
Physicochemical properties of PLGA nanoparticles: Particle size analysis and zeta potential were determined by Zetasizer Nano ZS (Malvern Instruments, UK). Additionally, the microscopic characteristics of the PLGA nanoparticles were investigated by Scanning Electron Microscopy (SEM, TESCAN Vega LMU, USA).
In vitro release of protein from nanoparticles: At first, 0.5 mg of the antigen were loaded on nanoparticles and were dissolved in 2 ml Phosphate Buffered Saline (PBS) buffer. The sample was then placed in an incubator shaker at 300 rpm at 37°C. Afterwards, the sample was centrifuged and 300 µl of the supernatant were removed and replaced with the same volume of fresh PBS at specified time intervals. The amount of the released recombinant protein was determined using Bicinchoninic Acid (BCA) protein assay kit (Abnova) 24.
Animal immunization: BALB/c mice (6-8 weeks) were used for immunization. The principles in the Guide for the Care and Use of Laboratory Animals were followed and all animal experiments were conducted in compliance with the Welfare Act and regulations related to experiments involving animals. This study was approved with registration number 13989407 by Ethics committee. For subcutaneous administration, a group of 20 mice received 20 µg of free rGroEL in PBS. The immunization was performed with two injections with a two-week interval. The second group received 20 µg of the recombinant protein encapsulated in PLGA. The third and fourth groups received 20 µg of Bovine Serum Albumin (BSA) and PLGA nanoparticles, respectively. Blood samples were taken from the mice before and after immunization.
Determination of anti-rGroEL IgG responses: Enzyme-Linked Immunosorbent Assay (ELISA) was used to determine the antibody titers. Maxisorb plates (Nunc) were coated with 5 µg of recombinant protein and were blocked with 5% skim milk in PBST (PBS plus 0.05% Tween 20). The washed plates were incubated with serially diluted serum samples at 37°C for 30 min. The plates were washed four times with PBST. Then, 100 µl of 1 in 2000 dilution of Horseradish Peroxidase (HRP)-conjugated goat anti-mouse IgG were added to each well plate. After that, the plate was incubated at 37°C for 30 min and was then washed four times with PBST. Meanwhile, the plates were incubated with HRP-conjugated anti-mouse monoclonal IgG1 or IgG2a under the same conditions. Then, 100 µl of the substrate solution containing 3 mg O-Phenylene Diamine (OPD) were added to each well plate. Finally, the reaction was stopped with 2.5 M H2SO4 and the absorbance was read at A495 nm on a microplate reader.
In vivo challenge studies: The mice groups were immunized with rGroEL, as described above. Fifteen days after the last booster, the mice were challenged intraperitoneally with 1 and 5 lethal doses of EPEC [1×107 Colony Forming Unit (CFU)/mouse, 5×107 CFU/mouse] and EHEC (1×108 CFU/mouse, 5×108 CFU/mouse), respectively. The mice were observed daily for morbidity and mortality for 14 days.
Organ burden: To assess the bacterial load in the control and immunized mice, spleen and liver were collected from individual animals. A tissue homogenizer was used to homogenize the tissues in 5 ml of ice-cold PBS. The resulting homogenates were plated in 10-fold serial dilutions on LB agar plates followed by incubation at 37°C for 18 hr. The CFU was counted and recorded.
Passive immunization: Groups of 10 mice were immunized passively by injecting 100 μl of heat inactivated hyper-immune mice serum intraperitoneally. The control (free polymer administration) mice were injected with 100 μl of heat-inactivated normal mice serum. After 24 hr, the mice were challenged with EHEC and EPEC intraperitoneally, as described earlier.
Statistical analysis: The normality of the data was assessed by the Kolmogorov-Smirnov test. Student’s t-test was used to compare the control and test groups. Additionally, repeated measurement tests were used to compare the effects of antigen administrations on the antibody titer. Statistical analyses were carried out by the SPSS 22.0 software.
Results :
Cloning of grol gene and preparation and characterization of the recombinant protein: The grol gene was cloned into a pET28a expression vector and was validated by restriction digestion analysis (Figure 1A). DNA sequencing with T7 and T7 term universal primers confirmed the cloned gene fragment. The recombinant vector was transferred to E. coli Rosetta strain and induced recombinant protein expression by IPTG. A 64.5 kDa protein band on SDS-PAGE showed a high level of recombinant protein expression (Figure 1B). Purification of the protein was carried out under native conditions, and SDS-PAGE analysis revealed the presence of the recombinant protein in the eluted fraction (Figure 2). The yield of recombinant protein production was 8.2 mg/L. The recombinant protein was verified with western blotting analysis (Figure 3).
Characterization of rGroEL-PLGA: PLGA nanoparticles were prepared by the double emulsion method. Based on the results, the loading efficiency of rGroEL-PLGA nanoparticles was 89.5±2.5% and the average particle size was 194.3±3.2 nm. The zeta potential of the nanoparticles was
-18.2±1.1. The external morphology of the nanoparticles studied by SEM revealed that the nanoparticles were approximately spherical in shape and had a smooth surface (Figure 4). Furthermore, in vitro release of chimeric protein from the PLGA in PBS exhibited an initial burst release followed by a sustained release of the protein (Figure 5).
Evaluation of serum antibody titer response against recombinant GroEL protein: To determine the antigen humoral responses primed by immunization with GroEL, IgG, IgG1, and IgG2a levels were determined in the sera of the immunized mice via ELISA. The results revealed a significant increase in IgG levels in the GroEL-PLGA immunized mice compared to the controls with free PLGA administration (p<0.05) (Figure 6). Anti-GroEL IgG titers were also noticed in the animals immunized with free GroEL. However, the titers were significantly lower compared to the GroEL-PLGA immunized animals. To reveal the type of immune response, IgG1 and IgG2a subclasses were determined. The results revealed a predominant IgG1 response. Nevertheless, significant IgG2a levels were detected in both GroEL-PLGA and free GroEL immunized mice (Figure 7).
In vivo challenge studies: Fifteen days after the last immunization, the mice were challenged with EHEC and EPEC. The results showed a significant difference between the control (free PLGA) and immunized groups regarding the number of survived mice (p<0.05). Accordingly, all the control mice died within four days of challenge with the above pathogens, whereas the GroEL immunized group showed 80% protection against the lethal infection by EPEC and 60% protection against the lethal infection by EHEC. When the challenge dose was raised to 5 LD, the protection rate against EPEC and EHEC decreased to 40 and 30%, respectively. No mice survived in the PBS groups when challenged with either the high or the low dose (Figure 8).
Organ burden: The bacterial organ load was estimated from the spleens and livers collected from different groups of mice challenged with different pathogens. The findings indicated a significant decrease in CFU in the livers and spleens of the GroEL immunized mice challenged with EPEC and EHEC compared to the control group (p<0.05) (Figure 9).
Passive immunization: To assess the role of humoral immunity in eliciting protection against lethal infection by EHEC and EPEC, the mice were immunized by injecting the heat-inactivated mice anti-GroEL antiserum. After 24 hr, the mice were challenged with EPEC and EHEC, as mentioned before. The results demonstrated that 50% of the animals that were passively immunized survived the lethal infection by EPEC or EHEC (Figure 10). However, when the challenge dose was raised to 5 LD, the protection rate against EPEC and EHEC decreased to 40 and 20%, respectively. No mice survived in the PBS groups when challenged with either the high or the low dose (Figure 10).
Discussion :
We evaluated the immune responses of full-length GroEL of S. typhi against EPEC and EHEC bacteria by encapsulation in PLGA. The increased antimicrobial resistance among EPEC and EHEC has limited the efficacy of traditionally used antibiotics 25,26. This has prompted the development and use of vaccines as an alternative key strategy to overcome the drug resistance 27. The HSPs elicit both humoral as well as cell-mediated immune responses with potential to develop as new generation prophylactic and therapeutic vaccines against infectious agents 20,25. In this context, the immune responses of GroEL from S. typhi against EPEC and EHEC could be a strategy of choice. Encapsulation of immunogens in PLGA has the advantage of retaining protein integrity from degradation in addition to the sustained immunogen release, and enhancement of both systemic and mucosal antibody responses. In the present study, we expressed and purified the recombinant GroEL in E. coli Rosetta. The protective efficacy of PLGA encapsulated recombinant GroEL was studied in murine model.
An initial burst release of antigen was observed shortly after incubation of nanoparticles under neutral pH conditions. Approximately 20% of the antigen was released soon after the incubation of the nanoparticles corresponding to a priming immunization dose. The initial burst effect is attributable to the rapid release of antigen located on or near the surface of the nanoparticles 8.
Slow diffusion into the medium of the entrapped protein from nano matrix brings about low release of the protein following initial burst release 23. Antigenicity of the released antigen determined by western blot analysis demonstrated that the chimeric protein remains intact retaining its antigenicity. The result of the present study revealed that the immunization of mice with PLGA encapsulated recombinant GroEL significantly increases IgG antibody titers in sera as compared to control groups. For a potential candidate vaccine molecule, the type of immune response induced is very important which is determined by the IgG1and IgG2a isotypes. Antibody isotyping revealed elevated levels of IgG1 and IgG2a antibodies in the test group as compared to the control group, indicating the induction of both Th1 and Th2 types of immune responses. These results were consistent with the previous report of a similar increase in IgG1, IgG2a levels in the sera of immunized mice with GroEL and Freund’s adjuvant 28.
The in vivo cross-protective efficacy of PLGA encapsulated GroEL of S. typhi in immunized mice was determined by challenging them with the EPEC and EHEC 80% and 60% protection was achieved against a lethal dose of EPEC and EHEC, respectively. This cross-protection indicates that the immune response is directed to the shared epitopes between these bacteria, suggesting the use of HSPs in the prevention of diseases caused by other pathogens viz.; Shigella flexneri, Shigella dysenteriae type I, Shigella boydii, Pseudomonas aeruginosa and Klebsiella pneumoniae. It was reported that S. typhi GroEL cross-protected against diarrheal disease induced by multiple pathogenic bacteria 26. Chitradevi et al investigated the potential for cross-protection of the recombinant GroEL of Salmonella enterica serovar typhi against EPEC. Their studies showed that Salmonella GroEL protected 75-80% of mice against EPEC 26. This is the first report of the efficacy of PLGA encapsulated S. typhi GroEL immunization against EPEC and EHEC. The protective mechanism could be mediated by induction of both humoral and cellular immune responses as revealed by our earlier studies 26.
There are two major limitations in this study that could be addressed in future research. First, EHEC and EPEC are gastrointestinal pathogens; therefore, induction of local mucosal immunity is expected to play an important role in protecting against these bacteria. The current experimental design and intraperitoneal injection mode of immunization with PLGA encapsulated recombinant protein, help to predict the feasibility of such approaches to induce pathogen-specific mucosal immunity. However, it is necessary to study the oral immunogenicity of nanoparticles in future studies. However, it should be noted that physiological mouse models of these diseases are not readily available. Second, one of the major limitations in using traditional E. coli strains relates to the LPS component of the outer membrane 29. In the present study, the recombinant GroEL was expressed in E. coli Rosetta and LPS content in the recombinant protein preparation was not measured. LPS acts as a natural adjuvant and stimulates T cell responses. This could contribute to the observed immune responses. Elimination of LPS contamination in future study is necessary to reduce the potential effect of LPS on induction of immune responses.
Conclusion :
In conclusion, the PLGA encapsulated GroEL is an interesting candidate for a subunit vaccine that offers cross-protection or enhances the immune system during infections caused by EPEC and EHEC.
Acknowledgement :
The authors would also like to appreciate Ms. A. Keivanshekouh at the Research Consultation Center (RCC) of Shiraz University of Medical Sciences for improving the use of English in the manuscript.
Funding: This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest :
The authors declare no conflict of interests.
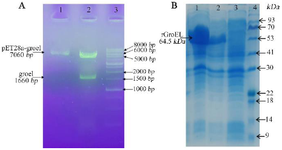
Figure 1. A) Confirmation of the recombinant plasmids by double-digestion on gel lectrophoresis. Lane 1: the purified plasmids of pET28-groEL (clone 1) on 1% agarose gel; Lane 2: the double digested products using BamH1/HindIII for pET28-groEL (~1664 bp); Lane 3: molecular weight marker (1 Kb DNA ladder). B) SDS PAGE analysis of groEL recombinant protein expression. Lanes 1, 2: total protein of two induced E. coli clones; Lane 3: total protein of non-induced cells; Lane 4: protein weight marker (Pr901641, Sina colon).
|
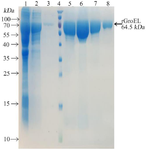
Figure 2. Purification of the recombinant protein through affinity chromatography using Ni-NTA resin columns. Lane 1: cell lysate; Lane 2: flow-through; Lane 3: column washed with imidazole 20 mM; Lane 4: protein weight marker (PageRuler™ Prestained Protein Ladder, 10 to 180 kDa, Thermo Fisher); Lanes 4-8: purified protein after elution with imidazole 250 mM.
|
Figure 3. Western blott analysis of the recombinant protein. Lane 1: Protein weight marker, (Sl7012, Sina colon). Lane 2: purified recombinant protein. Lane 3: BSA as control.
|

Figure 4. Physicochemical properties of PLGA nanoparticles. A) Scanning electron micrograph of PLGA nanoparticles encapsulated with recombinant protein; B) Size distribution of PLGA nanoparticles; C) Zeta potential distribution of PLGA nanoparticles.
|
Figure 5. In vitro cumulative release of the recombinant antigen from PLGA nanoparticles. Values have been expressed as mean±SD (n=3).
|
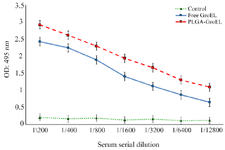
Figure 6. Detection of the specific IgG titers in the sera of the immunized mice
|
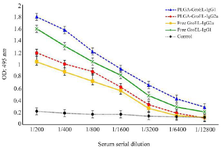
Figure 7. The effects of Free GroEL and GroEL-PLGA immunization on IgG1 and IgG2a antibody levels in the mice
|

Figure 8. The effect of GroEL immunization on the survival of the mice challenged with 1 and 5 lethal doses of EPEC and EHEC strains.
|
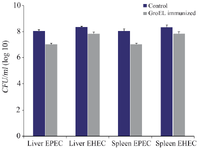
Figure 9. Liver and spleen organ burden estimated by the average CFU/ml in the control and GroEL immunized mice challenged with EPEC and EHEC. The bacterial CFU counting was done with three replicants. Bacterial CFU was presented as the mean values ± standard error (SE), and a p<0.05 was considered statistically significant.
|
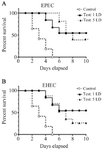
Figure 10. The protective efficacy of rGroEL immunization on the survival of the mice passively immunized with rGroEL. The mice were passively immunized with heat inactivated immune sera raised from the mice immunized with rGroEL. These mice were challenged with 1 and 5 lethal doses of EPEC and EHEC strains.
|
|