Investigation of Durability of SARS-CoV-2-specific IgG and IgM Antibodies in Recovered COVID-19 Patients: A Prospective Study
-
Zamani, Mohammad
-
Digestive Diseases Research Institute, Shariati Hospital, Tehran University of Medical Sciences, Tehran, Iran
-
Ghasemi, Ahmad
-
Healthy Ageing Research Centre, Neyshabur Faculty of Medical Sciences, Neyshabur University Medical Sciences, Neyshabur, Iran
-
Shamshirgaran, Morteza
-
Healthy Ageing Research Centre, Neyshabur Faculty of Medical Sciences, Neyshabur University Medical Sciences, Neyshabur, Iran
-
Ahmadpour, Sajjad
-
Gastroenterology and Hepatology Diseases Research Center, Qom University of Medical Sciences, Qom, Iran
-
Hormati, Ahmad
-
Gastroenterology and Hepatology, Department of Internal Medicine, School of Medicine, Gastrointestinal and Liver Diseases Research Center, Iran University of Medical Sciences, Tehran, Iran
-
Khodadadi, Javad
-
Infectious Disease Department, Qom University of Medical Sciences, Qom, Iran
-
Varnasseri, Mehran
-
Infectious and Tropical Diseases Research Center, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
-
Amini, Fatemeh
-
Department of Persian Medicine, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran
-
Shayanrad, Amaneh
-
Liver and Pancreatobiliary Diseases Research Center, Digestive Diseases Research Institute, Shariati Hospital, Tehran University of Medical Sciences, Tehran, Iran
-
Poustchi, Hossein
-
Liver and Pancreatobiliary Diseases Research Center, Digestive Diseases Research Institute, Shariati Hospital, Tehran University of Medical Sciences, Tehran, Iran
-
Shabani, Mahdi
-
Department of Immunology, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
Abstract: Background: Evidence on seroconversion profile of the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infected patients is limited. We mainly aimed to evaluate seroconversion and persistence of virus-specific antibodies in patients infected by coronavirus disease 2019 (COVID-19).
Methods: This prospective study was conducted on 118 patients with COVID-19 presentations admitted to three hospitals in Iran and recovered from the disease, during April and May 2020. Presence of COVID-19 was confirmed by Polymerase Chain Reaction (PCR) testing on nasopharyngeal swabs. Serum samples were collected at different time points, including 0-5, 6-15, 16-25, 26-35, and 36-95 days of clinical symptom onset. For measurement of SARS-CoV-2-specific IgG and IgM antibody titers, Iran's Food and Drug Administration-approved SARS-CoV-2 ELISA kits were used.
Results: Serologic assay revealed that 37.3% of patients (n=44) were positive for IgM at 0-5 days interval after clinical symptom onset. This rate was 60.2% (n=71) for IgG. There were increasing IgM and IgG seroconversion rates during first 25 days of clinical symptom onset, but seropositivity started to decrease thereafter, which was more evident for IgM (17.9%) than IgG (58.9%) at the 36-95 days post symptoms appearance. In other words, it was found that 83.6% of IgM-positive and 32.9% of IgG-positive patients in the first month of clinical symptom onset became seronegative in the third month of clinical symptom onset.
Conclusion: The findings demonstrated that antibody responses to SARS-CoV-2 infection were developed in recovered COVID-19 patients; however, some of them were seronegative three months after onset of relevant symptoms. Furthermore, the stability of anti-SARS-CoV-2 antibodies could also correct our expectations from COVID-19 vaccination responses.
Introduction :
The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes the corona virus disease 2019 (COVID-19), spread rapidly and infected millions of people worldwide during 2020-2021 1. The disease was declared as a pandemic by the World Health Organization (WHO) on 11 March 2020. So far, more than 5.6 million people have died from this infection around the world and this is increasing 2. The clinical presentations of COVID-19 vary from asymptomatic infection to a life-threatening acute respiratory failure requiring intensive care support. A recent meta-analysis also reported an average incubation period of 5-7 days for this disease 3. To diagnose the infection with SARS-CoV-2, current guidelines recommend to use a Real-Time reverse transcription Polymerase Chain Reaction (RT-PCR) assay on nasopharyngeal and oropharyngeal swabs. However, this method has shown false positive and negative errors in different settings 4. Furthermore, the availability and accessibility of molecular testing is a major concern to massive testing in population that underestimates the true number of infected cases. Thus, serologic testing could be an available option for this purpose, which also acquired the US Food and Drug Administration’s (FDA) emergency use authority for the diagnosis of SARS-CoV-2 on February 4, 2020 5. In a literature review of 54 published papers, the accuracy of IgM and IgG combination tests was 91.4% at 15-21 days after symptom onset 6.
Despite a faster response and less workload for serologic assay compared with PCR, there has been a challenge to interpret the antibody data due to the uncertain time course of antibody response and seroconversion. Some studies reported that SARS-CoV-2 specific IgG and IgM antibodies were observed in most of the patients after 7-14 days of clinical symptom onset 7,8. It was pointed out that relative sensitivity of one serologic assay with initial sensitivity of >95% decreased to 85 and 71% at 61-80 and 81-100 days after diagnosis of mildly COVID-19 symptomatic outpatient, respectively that could imply on instability of SARS-CoV-2 antibody responses 9. Altogether, more studies need to be done to provide conclusive evidence about profile of humoral responses to COVID-19 which is very critical for the interpretation of serological findings in this disease. Besides, there is uncertainty about the persistence of the antibody response to SARS-CoV-2. The perspective studies showing the long-term kinetics of antibodies to SARS-CoV-2 need to be performed to appropriately guide us to interpret serological findings.
In this study, we aimed to investigate the trends of seroconversion and durability of antibody response to SARS-CoV-2 in the patients with confirmed COVID-19 who were admitted to different hospitals and recovered from the disease. The results of the present study would hopefully be helpful to better understand the serological patterns of SARS-CoV-2 antibodies and the clinical value of antibody testing for the disease.
Materials and Methods :
Patients and samples
This prospective study enrolled 118 patients with COVID-19 presentations who were admitted to 22-Bahman Hospital in Neyshabur, Kamkar Hospital in Qom, and Razi Hospital in Ahvaz, Iran, during April and May 2020. Among them, 39 patients (33.1%) were hospitalized and 79 patients were outpatients. The cases whose symptoms (fever, respiratory, and/or gastrointestinal complaints) started within maximum 5 days were included in this study. COVID-19 diagnosis was based on both of clinical manifestations and real-time RT-PCR. The nasopharyngeal sampling was performed on all suspected cases. The swab sample was then put into a tube labeled with a unique participant identity number and stored frozen at -20°C until needed. Finally, a one-step real-time RT-PCR kit (Pishtaz Teb, Tehran, Iran) was used for nucleic acid detection of SARS-CoV-2 according to the manufacturer's protocol 10. In addition, in total, 394 serial blood specimens were also obtained from 118 patients with COVID-19 at different time intervals, including 0-5, 6-15, 16-25, 26-35, and 36-95 days of clinical symptom onset. From each patient, 5 ml of venous blood was collected into a tube labeled with a unique participant identity number. Then, the tubes were centrifuged with 3000×g for 10 min, to separate the serum. The obtained serum samples were stored frozen at -20°C until needed.
Serologic assay
For measurement of SARS-CoV-2-specific IgG and IgM antibody titers in serum samples, the Iran's Food and Drug Administration-approved-approved SARS-CoV-2 ELISA kits (Pishtaz Teb, Tehran, Iran) were used according to the manufacturer's protocol 11. The SARS-CoV-2 IgM ELISA kit was designed based on IgM-captured sandwich method to detect both nucleocapsid and spike S1 proteins of the virus; also, SARS-CoV-2 specific IgG to nucleocapsid was detected by indirect SARS-CoV-2 IgG ELISA kits. Briefly, one hundred µl of prepared serum specimens were added into appropriate wells and incubated for 30 min at 37°C. After washing, 100 µl of appropriate conjugates (anti-human IgM-HRP, or anti-human IgG-HRP) were applied into the wells and incubated for 30 min at 37°C. Then, 100 µl of chromogenic substrate was dispensed into the wells. All plates were incubated at room temperature and darkness for 15 min. By adding stop solution, the optical densities of the developed color in the wells were measured at 450 nm and 630 nm as the reference filter using ELISA reader (BioTek Instrument Inc., Winooski, VT, USA). To determine positive or negative results, the sample optical density was divided by the cut-off value (S/C index). A S/C ratio of >1.1 was considered positive.
Data analysis
After enrollment of the patients, the necessary demographic, laboratory, and clinical data were collected by a checklist form. Data analyses were conducted using SPSS software. Descriptive analysis was used to calculate frequency, percentage, mean and standard deviation. Mann–Whitney test was used to compare two groups of nonparametrically distributed data. Also, Spearman correlation test was used to assess the correlation between age and antibody titers. Levels (S/C) of IgM and IgG antibodies against SARS-CoV-2 were presented by scatter plots. A p-value less than 0.05 was considered statistically significant.
Results :
In total, 394 blood specimens were obtained from 118 recovered COVID-19 patients. Of these patients, 93 (78.8%) were from Neyshabur, 13 (11%) were from Ahvaz, and 12 (10.2%) were from Qom cities of Iran. Out of 118 patients, 46 (39%) were male and 72 (61%) were female. The mean age of the participants was 46.86±15.84, ranging from 18 to 89 years old. Demographic and clinical characteristics of patients are presented in table 1.
The serologic assay at 0-5 days after clinical symptom onset showed that 37.3% of the patients (n=44) tested positive for IgM. This rate was 60.2% (n=71) for IgG at that time-points. IgG seropositivity in different intervals was at higher rates for all time points of testing compared with IgM positivity (Table 2). Also, the peaks of IgM (68.6%) and IgG (80%) seroconversion were observed in the first 25 days of symptom onset, but seropositivity started to decrease thereafter (Figure 1, Table 2). A slight drop was seen for IgG positivity from the 16-25 days interval (80%) to the third month of clinical symptom onset (58.9%), while this decreasing trend was more evident for IgM positivity (from 68.6% to 17.9%). It was found that 83.6% (n=46) of 55 IgM-positive patients and 32.9% (n=23) of 70 IgG-positive patients in the first month of clinical symptom onset became seronegative in the third month of clinical symptom onset (Figure 2).
In accordance to the seropositivity rate, IgG titers were also higher than IgM titers in all defined time intervals with a decrease of the antibody titers after 15 days of symptoms onset (Figure 3). No correlation was found between age and antibody titers at any of the defined time points. Also, no significant differences were identified between sex groups in the mean of antibody levels at different times after clinical symptom onset.
Discussion :
Currently, RT-PCR is a diagnostic method for confirmation of SARS-CoV-2 infection; however, it has some limitations that make it less ideal in practical scenarios. Therefore, researchers are working on antibody testing as an accompanying method. In addition, the serological profile of specific antibodies to SARS-CoV-2 has great impact on applicability and interpretation of serological findings in COVID-19. In this study, we attempted to provide evidence on antibody responses and seroconversion, as well as the durability of humoral IgM and IgG to SARS-CoV-2, in recovered COVID-19 patients. According to the results, the positivity rate of IgM and IgG antibodies increased over the first 25 days after clinical symptom onset, and then started to decrease gradually. Of course, the seropositivity rate plateaued for IgG antibody from 16 days after clinical symptom onset for ten days, and then mildly decreased. For IgM antibody, the seropositivity was sharply reduced from 26-35 days after clinical symptom onset to the last follow-up (three months after clinical symptom onset). The study by Long et al 12 showed that IgG seroconversion started from the first days of clinical symptom onset and was seen in all patients beyond 17–19 days, while IgM positivity reached a peak of 94.1% in 20–22 days. The peak times in Long et al’s study for IgM and IgG seroconversion were almost similar to our study; however, the seroconversion rates in their study were different from ours. This difference might relate to the specificity and sensitivity of the kits used in the study. Also, Phipps et al 8 stated that IgG seroconversion rate increased from initial testing (7%) to two weeks after clinical symptom onset (83%), which was consistent with our results. However, the authors observed no increase in IgM positivity within the first two weeks. In their survey, Fu et al 13 alluded to the seroconversion rate of 19.3% for IgM in the first week of clinical symptom onset, reaching a peak of 81.5% in the fifth week, and then declining to 55% in 9-10 weeks. Regarding IgG seroconversion, the authors reported a rate of 44.6% in the first week that reached 93.3% in the fourth week and remained high thereafter. Altogether, there are differences between various studies in seroconversions rates, which can be due to different populations, disease severity of included subjects, uncertain dates of infection, varied ELISA kits, follow-up periods, and so on. However, it can be concluded that the viral load is likely maximized in the early phase of the disease and serologic testing probably has better performance in 2-4 weeks after onset of COVID-19 symptoms in order to help COVID-19 diagnosis and follow up.
As mentioned above, the percentage of the patients tested positive for IgG was higher than those tested positive for IgM at 0-5 days after clinical symptom onset (60.2 vs. 37.3%). Similar to our results, Long et al 12 reported that IgG seropositivity was higher than IgM seropositivity in the first days of clinical symptom onset; they also observed that some patients were IgG-positive but IgM-negative. This could be partially explained by the possible differences in the diagnostic accuracy of the kits used for measurement of the antibodies. Another likely explanation could be that in some patients formerly exposed to other species of coronavirus (e.g., human coronavirus OC43), plasma cells and memory cells released IgG antibody against the similar epitopes on the SARS-CoV-2 (cross-reac-tivity), leading to a higher rate of IgG seropositivity than IgM seropositivity.
Regarding the stability of antibody positivity, we compared the number of patients who seroconverted in the first month after clinical symptom onset (at any time of testing) vs. those who seroconverted in the third month after clinical symptom onset. It was found that more than four-fifth of IgM-positive patients became seronegative, which was much more than the rate of IgG negativity (33%). Both virus-specific IgM and IgG antibody levels increased from the first serologic testing and peaked about two weeks after clinical symptom onset, and then steadily decreased. Liu et al 14 showed that SARS-CoV-2 specific IgG peaked at 25 days and were still stayed stable at high level 1 month after disease onset. However, the profile of IgM to SARS-CoV-2 had a different pattern that disappeared 1 month after onset of symptoms either in mild and severe COVID-19 patients. The IgG titer decline shows different rates in asymptomatic and symptomatic patients. In an elegant study, Long et al 15 reported that 40% of asymptomatic individuals and 12.9% of the symptomatic patients became seronegative for IgG in the early convalescent phase (8 weeks after discharge). A research from Iceland demonstrated that Pan-Ig antibodies to SARS-CoV-2 remained positive at 4 months following the diagnosis. However, the scenario was different for Ig subclasses 16. In this regard, the level of nucleocapsid specific IgM rapidly fell and generally became negative after 2 months. The anti-nucleocapsid and anti-spike S1 IgG titer increased at 6 weeks after diagnosis but decreased slightly after then 16. In addition, Adams et al 17 also pointed out that IgG titer raised in the first 3-week interval after onset of symptoms and then decreased during the second month of COVID-19 symptoms presentation, although remained above the defined optical density threshold. These published works expressed the mid-term stability of IgG to SARS-CoV-2 during a natural infection that could be different from vaccine-induced antibody response. Decline in SARS-CoV-2 IgM titer could be correlated to the short-lived IgM producing memory cells as the origin source of this class of Ig. This point has been declared in a recent work that indicated memory B cells of COVID-19 patients mainly belongs to IgG memory B cells with a minor population of IgA memory B cells 18. It is noteworthy that antibody detection to SARS-CoV-2 could not simply predict the durability of immune memory in recovered COVID-19. In this regard, persistence of memory B cells, memory CD4+ T cells, and memory T follicular helper cells in 95% of recovered COVID-19 patients during 6 months after the infection could be considered as the stability of immune memory response to SARS-CoV-2 18. Thus, clear understanding about kinetics and durability of humoral responses to SARS-CoV-2 will help us to better predict the applicability of serological testing for improving COVID-19 diagnosis, as well as our expectation on vaccination responses.
In the present study, we used commercial ELISA kits for measurement of SARS-CoV-2 specific antibodies. For IgG, the test detected the level of antibody against the viral nucleocapsid antigen; considering that IgG antibodies are mostly produced against the nucleocapsid protein 19, detection of anti-spike S1 protein antibodies as an additional method seems not to increase the diagnostic accuracy (data not presented). On the other hand, for IgM, we conducted the antibody capture assay detecting both of nucleocapsid and spike S1 proteins of the virus.
A limitation of the present study was lack of referral of some patients for serologic tests at uncertain times after infection that led to missing data. Also, some patients were not tested consistently at regular intervals.
In addition, we were not able to increase the sampling time, due to limitations in patients’ referral and their inaccessibility, as well as sampling costs; however, it is suggested performing new studies with extended follow-up (up to 12 months). Finally, the virus neutralization, and therefore, the neutralizing activities of the antibodies were not evaluated.
Conclusion :
Findings of the present study demonstrated that antibody responses to SARS-CoV-2 infection were wildly induced in recovered COVID-19 patients. There were increasing IgM and IgG seroconversion rates during the first 25 days of clinical symptom onset, but positivity started to decrease thereafter. A slight drop was seen for IgG seropositivity from the first 16-25 days after clinical symptom onset to the third month of clinical symptom onset, while this decreasing trend was more evident for IgM seropositivity. Our results also help to identify optimal time periods for antibody testing on suspected cases, that is, it can be concluded that serologic testing probably has better performance in early months of clinical symptom onset. Altogether, serologic tests can be offered as a method besides molecular tests to support the diagnosis of acute SARS-CoV-2 infection. Clear understanding about stability of antibody responses will help us to better predict the applicability of serological testing for improving COVID-19 diagnosis as well as our expectation from vaccination responses.
Acknowledgement :
We are thankful to Pishtaz Teb Zaman Diagnostics for providing us with the SARS-CoV-2 IgM and SARS-CoV-2 IgG ELISA testing kits. We also thank the staff of the hospitals (such as physicians who contributed in the diagnosis and management of the cases) and the research centers (who contributed in the sampling process).
Conflict of Interest :
All authors declare no conflict of interest.
Ethical Approval :
After initial explanation of the study details to the patients, the written informed consent was taken from all of them. The patients’ information was also kept confidential. The study protocol was approved by the ethics committee of Tehran University of Medical Sciences (IR.TUMS.VCR.REC.1399.325).
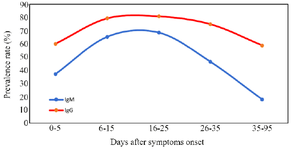
Figure 1. Seropositive profile of SARS-CoV-2 virus-specific IgG and IgM versus days after clinical symptom onset.
|
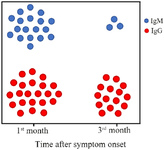
Figure 2. Stability of antibody seroconversion over the time after clinical symptom onset. Colored circles represent relatively the patients who were antibody-positive in the first month of clinical symptom onset (at any times of testing) versus those who were antibody-positive in the third month of clinical symptom onset.
|
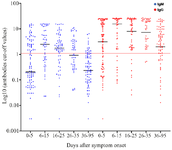
Figure 3. Levels of SARS-CoV-2 IgM and IgG antibodies in recovered COVID-19 patients at different times after clinical symptom onset. The scatter dots denote cut-off values of IgM and IgG antibodies of each sample. The red horizontal line defines cut-off value to separate IgM and IgG positive and negative samples.
|
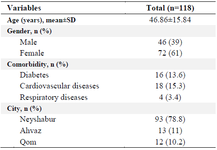
Table 1. Demographic and clinical characteristics of recovered COVID-19 patients included in the study
|
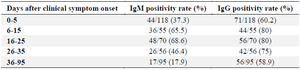
Table 2. Seropositive rates of SARS-CoV-2 virus-specific IgG and IgM in different time intervals after clinical symptom onset
|
|