GC/MS Analysis and Phyto-synthesis of Silver Nanoparticles Using Amygdalus spinosissima Extract: Antibacterial, Antioxidant Effects, Anticancer and Apoptotic Effects
-
 Hamdi, Seyed Mohammad Mahdi
Department of Biology, Central Tehran branch, Islamic Azad University, Tehran, Iran, m.hamdi@iauctb.ac.ir
Hamdi, Seyed Mohammad Mahdi
Department of Biology, Central Tehran branch, Islamic Azad University, Tehran, Iran, m.hamdi@iauctb.ac.ir
-
Department of Biology, Central Tehran branch, Islamic Azad University, Tehran, Iran
-
Mirzaee, Amir
-
Department of Biology, Roudehen branch, Islamic Azad University, Roudehen, Iran
Abstract: Background: The present study was aimed at phyto-synthesized silver nanoparticles (AgNPs) using Amygdalus spinosissima (A. spinosissima) extract and to investigate the antibacterial, antioxidant effects, anticancer and apoptotic effects of phyto-synthe-sized AgNPs.
Methods: The bio-fabricated AgNPs were characterized using UV-visible spectroscopy (UV-visible), X-ray Diffraction (XRD), Fourier Transform Infrared (FTIR), Transmission Electron Microscopy (TEM), Scanning Electron Microscopy (SEM) and Energy Disper-sive X-ray (EDX).
Results: The phyto-synthesized AgNPs showed maximum absorption in 438 nm, in the UV-visible spectrum. XRD peaks were observed at 2θ values in 38.20°, 44.40°, 64.60°, and 77.50° which are indexed as (111), (200), (220), and (311) bands of Face-Centered Cubic (FCC) structures of silver. FTIR analysis indicated that the AgNPs were capped with A. spinosissima extract. SEM and TEM micrographs revealed that the fabricated AgNPs were spherical and the average size range was 17.89 nm. Also, the EDX results show that the content of Ag was 90%.
Conclusion: The phyto-synthesized AgNPs had significant antibacterial activity against Gram-negative bacteria, as well as, the AgNPs exhibited great inhibitory effects on DPPH radicals and their antioxidant properties were favorably comparable to the antioxidant outcomes of ascorbic acid. Moreover, the AgNPs showed anti-cancer activity against the MCF-7 cell line with the IC50=6.1 µg/ml. Moreover, the phyto-synthesized AgNPs could induce apoptosis in the MCF-7 cell line significantly. The GC-MS analysis of the A. spinosissima extract showed that 102 bioactive phyto-chemical compounds, which be of use to the synthesis of AgNPs.
Introduction :
Recently, nanoparticles have attracted researchers due to their many applications in drug delivery, micro-bial diseases and cancer management 1,2. Due to their small size (1 to 100 nm), these structures have unique physicochemical and biological properties 3. Among nanoparticles, metal nanoparticles are synthesized by physical and chemical methods, which are very im-portant 4. In chemical synthesis of metallic nano-particles, chemical reducing agents including sodium hydrochloride and hydrazine can reduce the metallic ions into metallic nanoparticles 5. However, a small number of substances used in the chemical methods are toxic and dangerous and the synthesized nanoparticles lack biocompatibility and it is always possible that they are not physiologically suitable for laboratory tests 6.
Despite all the studies on the antimicrobial pro-perties of the extracts, which have made them very suitable for use, some stimulant and toxic properties have been found in them, which create many limit-ations in their use. Silver nanoparticles alone is toxic and carcinogenic and should be used with caution during testing. However, the aim of this study is to synthesize plants with silver nanoparticles, which is not harmful to the environment, but can be dangerous for the researcher during the experiment, which must be observed during safety tests 4. To overcome these prob-lems, green synthesis or Phyto-synthesis methods are used in the fabrication of nanoparticles that are econo-mically feasible and environmentally friendly 7.
In the Phyto-synthesis of metal nanoparticles, the plants extract is used as a reducing agent instead of toxic chemical compounds 8. The plants extract acts as a stabilizing agent in addition to its reducing properties 9. The synthesis of metal nanoparticles using plant ex-tract is one-step and the plant extracts have secondary metabolites that can be caused by reducing properties 10. One of the metallic nanoparticles that are important in medical applications and today is synthesized by plant extracts is silver nanoparticles (AgNPs) 11. So far, many plant extracts are used for the synthesis of AgNPs, but, in this study Amygdalus spinosissima (A. spinosissima) extract we used for the synthesis of AgNPs. In relation to previous studies, the synthesis of silver nanoparticles using European marjoram plant and its antimicrobial effects can be mentioned. In this study, European marjoram extract was used as a re-ducing agent for the biological production of silver nanoparticles and their study showed that silver nano-particles had antimicrobial activity against gram-posi-tive and negative bacteria 12.
A. spinosissima is a species of tree native to Iran, Middle East and Central Asia, but widely cultivated elsewhere. A. spinosissima is a rich source of vitamin E, dietary fiber, B-vitamins, essential minerals mono-unsaturated fats and phytosterols which have cholesterol-lowering properties 13. Moreover, A. spinosissima has different biological properties including antibacterial, anticancer, antioxidant, antidiabetic, antiemetic, antihypertensive, hypoglycemic, hypolipidemic 14.
There were several reasons for choosing A. spinosissima in the present study. One of the useful areas for studying medicinal plants is to study their antimicrobial effects. As a result of the increasing use of antibiotics, many bacteria have become resistant to these drugs. Therefore, there is an urgent need to find new antimicrobial sources. And the use of medicinal plants for this purpose is a priority. Also, the effective antimicrobial compounds in them are less harmful to the consumer than antibiotics. Medicinal plants have also always been considered as chemical substitutes due to their ease of access, lower side effects, low toxicity and low cost 12.
More studies have shown that the Amygdalus species are rich in tannins, phenols, flavonoids, carotenoids, and alkaloids, and it has been reported to show high antioxidant activities and that it can be considered as major factor for reducing silver to AgNPs 15. This study was aimed to synthesize silver nanoparticles AgNPs using A. spinosissima extract and to investigate the antibacterial, anticancer and apoptotic effects of synthesized AgNPs.
Materials and Methods :
Plant collection and extract preparation
The fresh aerial parts and root of Amigdalus spinosissima (Bunge) French were obtained from the Iranian Biological Research Center (http://www.en.ibrc. ir/), and verified by Botanical Faculty (Herbarium no. 1391). To prepare the extract, the root and aerial parts of A. spinosissima were first placed in an air current and then completely dried in dark. 50 g of the powdered root and aerial parts, was added to 500 ml ethanol and distilled water solution for 12 hr and the resultant extract was stored at 4°C until used for the synthesis of AgNPs 16.
GC/MS analysis of A. spinosissima extract
The chemical composition of A. spinosissima extract was studied using a Gas Chromatography-Mass Spectrometer (GC/MS) system. GC/MS analysis was done on Schimadzu 15A gas chromatograph equipped with a split/spitless injector (250°С) and a flame ionization detector (250°С). Nitrogen was used as carrier gas (1 ml/min) and the capillary column used was a DB-5 (50 m 0.2 mm, film thickness 0.32 μm). The column temperature was kept at 60°С for 3 min and then heated to 220°С with a 5°С/min rate and kept constant at 220°С for 5 min. Relative percentage amounts were calculated from the peak area using a Shimadzu C-R4A Chromatopac, without the use of correction factors.
Phyto-synthesis and characterization of silver nanoparticles (AgNPs)
The AgNPs were phyto-synthesized by adding 100 ml of 1 mM silver nitrate with 5 ml of extract and incubated at room temperature for 30 min. After 45 min of reaction, the sediment was washed three times with distilled water by centrifugation at 13,000 rpm for 20 min, and the product was kept at 60°C for 2 hr 17. The absorption spectrum of prepared AgNPs was measured using a UV-vis spectrophotometer in the wavelength range of 200-700 nm. The dried powder of AgNPs was further analyzed by XRD (X-ray diffraction) and Fourier Transform Infrared spectrophotometer (FTIR). Size, shape, and morphology of the AgNPs were determined by Scanning Electron Microscopy (SEM) (XL30 electron microscope, Phillips, Japan) and Trans-mission Electron Microscopy (TEM) (Leo 906, TEM model 100 KV, Zeiss, Germany). The Energy-Disper-sive X-ray Spectrometer (EDX) analysis was perform-ed using the EDS X Sight Oxford instrument.
Radical scavenging activity (DPPH) test
The free-radical scavenging activity test was performed using suspensions of the synthesized AgNPs at various concentrations as well as DPPH (1,1-diphenyl-2-picrylhydrazyl). A 0.1 mM solution of DPPH in methanol was prepared, and 1 ml of this solution was added to 3 ml of nanoparticle suspension with concentrations: 5, 25, 50, 100, and 200 μg/ml. The reaction mixture was then vortexed thoroughly and left in the dark at room temperature for 30 min whose absorbance was measured at 517 nm using a spectrophotometer.
The DPPH radical scavenging ability of the AgNPs
was calculated as:
DPPH scavenging effect (%) = [(A0 – A1)/A0] × 100
Where, A0 and A1 represent the absorbance of the control and the sample, respectively. Subsequently, the concentration of AgNPs having a 50% radical scavenging activity was calculated and reported as IC50. In addition, ascorbic acid was used as a positive control in this test.
Antibacterial activity of AgNPs
To evaluate the antimicrobial properties of the phyto-synthesized AgNPs, a Minimum Inhibitory Concentration (MIC) method was used. At first, 4 pathogenic bacteria including Escherichia coli (E. coli) ATCC 25922, Salmonella enterica ATCC 14028, Staphylococcus aureus ATCC 25923 and Streptococcus pyo-genes ATCC 1447 were cultured in Luria-Bertani broth for 24 hr. The various concentrations of AgNPs including 100, 50, 25, 12.5, 6.25, 3.125 and 1.56 µg/ml was prepared and poured into the wells of the microplate. Subsequently, bacterial suspension with 0.5 McFarland concentration was added to each well. MIC is defined as the lowest concentration that inhibits microbial growth 18. Besides, the Minimum Bactericidal Concentration (MBC) is considered the lowest concentration of an antibacterial agent required to kill selected bacteria.
MTT assay
The MTT [3-[4,5-dimethylthiazol-2-yl]- 2,5-diphe-nyltetrazolium bromide] colorimetric method was used for determination of AgNPs cytotoxicity against breast cancer cell line (MCF-7). At first, 1×104 cells were seeded into 96-well plates, followed by incubation in a CO2 incubator at 37°C for 24 hr. The attached cells were treated with 100, 50, 25, 12.5, 6.25, 3.125, 1.56, 0.78 and 0.39 μg/ml concentrations of AgNPs for 24 hr. Subsequently, the MTT (Sigma Aldrich, Germany) was added into wells and the reaction mixture was kept at 37°C in a 5% CO2 atmosphere for 4 hr. The MTT dye was then removed and all wells were dissolved using isopropanol. Finally, the absorbance of the samples was measured using an ELISA Reader (Organon Teknika, Netherlands) at 570 nm and the cell toxicity was determined using the following formula 19:
% Cell cytotoxicity=100-[(At-Ab)(Ac-Ab)×100
% Cell survival viability=(At-Ab)(Ac-Ab)×100
% Cell inhibition = 100-cell survival
At=Absorbance value of test compound
Ab=Absorbance value of blank
Ac=Absorbance value of control
Apoptosis/necrosis assay
The flow-cytometric method was used to determination of apoptosis in MCF-7 cells treated with AgNPs. At first, 100,000 cells were treated with IC50 concentration of AgNPs and untreated cells were used as controls. Finally, the apoptosis/necrosis assay was performed using the Annexin V/Propidium Iodide (PI) kit according to the instructions 20.
Apoptosis gene expression
Bax and Bcl2 apoptosis genes expression were measured by Real-time qRT-PCR. Target gene expression levels were normalized to GAPDH as a housekeeping gene. The primers sequence of the target genes, including Bax and Bcl–2, and GAPDH (internal control), are given in table 1. Real-time qRT-PCR was performed independently in triplicate, for each of the different samples, and the data are presented as the mean values of the gene expression levels measured in the treated samples comparing to the controls 21.
Statistical Analysis
All assays were conducted in triplicate and the obtaining data were analyzed using a one-way analysis of variance (ANOVA) with the post hoc test employing SPSS 17.0 software and the results were expressed as mean±standard deviation (SD) of three replicates. Moreover, p<0.05 was considered as significant.
Results :
GC/MS analysis of A. spinosissima extract
Figure 1 shows the GC/MS analysis of all chemicals clarified in the A. spinosissima aerial part ethanolic extract. Based on table 1, 102 compounds were identified in the A. spinosissima extract. As mentioned earlier, A. spinosissima extract is full of polyphenolic compounds with different anti-oxidant, anti-cancer and anti-bacterial properties (Table 2) 13. The biological activity of major phyto-components identified in the extract is shown in table 2. Because of reducing and capping agents of the main phytochemical constituents in A. spinosissima extract, a cap was formed around Ag+ of the bio-functionalized A. spinosissima AgNPs which was stable.
Phyto-synthesis and characterization of AgNPs
After 60 min of the reaction and change of reaction color, the ultraviolet spectroscopy analysis of AgNPs was evaluated using a UV-vis spectrophotometer between 200 and 700 nm. The absorption of AgNPs was observed in 375 nm wavelength in the UV-vis spectrum. Moreover, no additional peaks were seen in the spectrum, which showed a pure synthesis of AgNPs (Figure 2). Photograph of TEM shows that the synthesized AgNPs are spherical with 17.89 nm average size and to confirm the size of these nanoparticles, they were re-examined by DLS. In addition, the size and morphology of biosynthesized AgNPs using SEM revealed that the fabricated AgNPs had a spherical structure (Figure 3). The XRD patterns of phyto-synthesized AgNPs are shown in figure 4. In comparison with the cubic Ag reference pattern, the spectra data reveal the five diffraction lines (111), (200), (222), (311), and (222) which are compatible with the standard pattern (Figure 5). EDX analysis was used to confirm the pres-ence of elemental silver in Phyto-synthesized AgNPs (Figure 6). FTIR analysis of AgNPs shows the surface AgNPs of extract, which are considered as reducing and capping agent. The peak at 1222 cm-1 represents C-N stretch. The peak at 1708 cm-1 shows C=O stretch. The peak at 2796 cm-1 represents H-C=O:C-H stretch (Figure 4).
DPPH free-radical scavenging assay
The antioxidant activity of the synthesized AgNPs was evaluated by DPPH radical scavenging assay, while ascorbic acid was used as a positive control. The AgNPs exhibited potential free radical scavenging activity (inhibitory concentration 50% [IC50]=51.22 μg/ ml). Note that the IC50 value for ascorbic acid was 9.35 μl/ml.
Antibacterial activity of AgNPs
In this study, the MIC method was used to study the antibacterial activity of phyto-synthesized AgNPs. In this test, pathogenic bacterial strains were treated with various concentrations of phyto-synthesized AgNPs including 0.78 to 100 μg/ml. The results of MIC and MBC showed that the biosynthesized AgNPs had the greatest effect against Gram-negative bacteria compared to Gram-positive bacteria (Table 2).
Cytotoxicity of AgNPs
The cytotoxicity of Phyto-synthesized AgNPs on MCF-7 cancer cells and L929 normal cells were evaluated using the MTT method and cell viability was measured after 24 hr. Treatment of both tumor and normal cell lines with various concentrations for 24 hr showed that the cytotoxicity of the synthesized AgNPs was dose-dependent while being less-toxic toward the L929 normal cell line. The results demonstrated that the bio-fabricated AgNPs exhibited the highest inhibitory effect on MCF-7 cells with IC50=6.1 µg/ml (Figure 7).
Apoptosis gene expression
Some molecular factors such as Bcl-2, Bax, casp3 and casp9 play a key role in the execution of apoptosis. Thus, to understand the molecular mechanism by which AgNPs induce apoptosis in MCF-7 cells, the expression ratios of Bax, Bcl–2, casp3 and casp9 genes in the MCF-7 cells treated with AgNPs were evaluated after 24 hr. Our results show that the expression of Bax, casp3 and casp9 genes, relative to the reference gene (GAPDH), was upregulated in the MCF-7 cells treated with AgNPs in 24 hr (3.26±0.56, 3.61±0.67 and 3.9 2±0.79, respectively); whereas the expression of anti-apoptotic Bcl-2 gene was significantly down-regu-lated (0.29±0.44) in MCF-7 cells treated with AgNPs (Figures 8 and 9).
Apoptosis/necrosis assay
To determine apoptosis induction in MCF-7 cells treated with an IC50 concentration of the AgNPs, the MCF-7 cells were stained with FITC Annexin V and PI and analyzed using flow cytometry. During the initial phases of apoptosis, phosphatidylserine is transferred outside the cell membrane and is thus stained with Annexin V; whereas the PI stain is attached to the nucleus during necrosis. The flow cytometry results are shown in Figure 10, where the upper left square (Q1) indicates the percentage of late apoptotic cells and the upper right square (Q2) represents the percentage of cells with early apoptosis. As the results indicated, the phyto-synthesized AgNPs have led to 54.70% early apoptosis and 8.04% late apoptosis in the treated cells.
Discussion :
Recently, plant extracts are widely used for the green biosynthesis of nanoparticles. Compared with micro-organisms as reducing agents, the use of plant extracts is fast, cost-effective, simple, non-pathogenic and convenient. Besides, the phytochemical compounds in the plant extract enhanced the activity of AgNPs. Among the secondary metabolites, polyphenols are considered as potent antioxidant compounds that cap-ture the ability of free radicals. If polyphenols are high in plant extracts, it will increase its antioxidant pro-perties and increase the intrinsic activity of AgNPs 21. In this study, we used the A. spinosissima extracts to synthesize AgNPs. The results also showed that synthesized AgNPs have significant antibacterial effects on Gram-negative bacteria so that AgNPs can alter the permeability of the cell membrane. Moreover, the AgNPs showed anticancer activity against the MCF-7 cell line with the IC50=6.1 µg/ml. Moreover, the phyto-synthesized AgNPs could induce apoptosis in the MCF-7 cell line significantly.
So far, several studies have examined the green synthesis of nanoparticles using plant extracts. In 2009, in a study the interaction of silver nanoparticles with the anti-tumor drug Aminocaracidine 9 was performed on cells. They showed that silver nanoparticles could control the release of the aminocaracidine 9 in living cells. They found that silver nanoparticles could act as a liberating drug carrier 22. In another study, Ahmed and his colleagues obtained a formulation and evaluation of azathioprine drug loaded on silver nanoparticles. Their method was green synthesis based on polysaccharides 23. Another study evaluated the loading of turmeric on silver nanoparticles for pharmacodynamics and antibacterial properties. The results of their research show-ed a high level of antibacterial properties 24.
The AgNPs were synthesized using A. spinosissima extract at 37°C, while other studies used high temperature and pressure to regenerate AgNPs. A. spinosissima extract has various phytochemicals that can reduce the silver ions. It was proposed that the phytochemical compounds containing hydroxide groups are responsible for the reduction of silver ions. Nanomaterials, and especially metallic nanomaterials, disable enzymes and DNA micro-organisms with electron equilibrium between electron donor groups such as thiol, carboxylate, amide, imidazole, endol, and hydroxyl due to their surface charge and surface-to-volume ratio. In this study, after 30 min of the addition of A. spinosissima extract, the silver ions were reduced and color changed, which represented the synthesis of AgNPs. The results of TEM and SEM showed that the phyto-synthesized AgNPs were spherical with 17.89 nm average size. In this study, two biological activities of AgNPs were studied: anti-microbial and cytotoxic activity. Antibacterial activity of phyto-synthesized AgNPs against pathogenic bacteria showed that AgNPs have significant antibacterial effects against studied pathogenic bacteria. Among these, E. coli and Salmonella were more sensitive to AgNPs comparing to others. More studies show that AgNPs have significant effects on Gram-negative bacteria comparing to Gram-positive bacteria 25.
One of the antibacterial mechanisms of AgNPs is the binding of AgNPs to the surface of the cell membrane that changed the permeability and cellular respi-ration. Another mechanism is the entrance of AgNPs inside the cell and the release of silver ions from AgNPs that bind to oxygen, sulfur, and nitrogen in functional biomolecules that lead to cell death 26. Another reason for more antimicrobial effects of AgNPs against Gram-negative bacteria is due to the lesser thickness of the peptidoglycan in Gram-negative bacteria 27.
Also, the results of this study showed that cytotoxicity of AgNPs is dose-dependent and it has significant effects on MCF-7 cancer cells compared to normal L-929 fibroblast cells. Till now, several studies have shown that AgNPs enter into cells through processes such as endocytosis, phagocytosis, and diffusion 28. Studies have also shown that the absorption of AgNPs depends on the cell type and the size of the nanoparticle 29. Miethling-Graff et al reported the size- dependent (10, 20, 40, 60, and 100 nm) effects of AgNPs in the human LoVo cell line 30.
Generally, cancer is one of the devastating diseases and its treatment is limited, but with the development of nanotechnology, the therapeutic application of AgNPs is under investigation 31. Moreover, studies show that cancer cells are more sensitive to AgNPs than normal cells 32. The AgNPs are effective in breast cancer, leukemia, hepatocellular carcinoma, skin and oral carcinoma 33. Finally, to investigate the mechanism by which AgNPs elicit cell apoptosis in a dose-dependent manner, Real-Time PCR was used in MCF-7 cells treated with an IC50 concentration of AgNPs. Our findings indicated that the expressions of apoptosis-related genes were increased with AgNPs treatment. Previous research reported that many different NPs and nanomaterials can affect apoptosis-related genes, such as Bax, casp3, casp9, and P53 34. Therefore, our Real-Time PCR data suggest that the increased apoptosis of AgNPs-treated MCF-7 cells was at least in part mediated by the change of apoptosis gene expression. Moreover, the flow-cytometric data support the apoptotic effect of AgNPs in the MCF-7 cell line. The MCF-7 cells exposed to AgNPs significantly increased the late apoptotic and necrotic cells as compared with untreated control cells.
Conclusion :
Generally, the results of this study showed that the phyto-synthesis of AgNPs are economical, eco-friendly and straightforward reproducible. The phyto-synthe-sized AgNPs had significant antibacterial activity against Gram-negative bacteria, as well as, the AgNPs exhibited great inhibitory effects on DPPH radicals and their antioxidant properties were favorably comparable to the antioxidant outcomes of ascorbic acid. Moreover, the AgNPs showed anticancer activity against the MCF-7 cell line with the IC50=6.1 µg/ml. The phyto-synthesize AgNPs were characterized by UV-Visible, EDX, XRD, FTIR, SEM, TEM. In FTIR, several phytochemical compounds identified which responsible for the reduction of silver ions to AgNPs. The results also showed that synthesized AgNPs have significant antibacterial effects on Gram-negative bacteria so that AgNPs can alter the permeability of the cell membrane. In addition, the phyto-synthesized AgNPs exhibited selective cytotoxicity toward the cancerous MCF-7 cell line when compared to their effect on the normal cell line tested, whereas, treatment of MCF-7 cells with AgNPs increases the expression of apoptosis genes including, Bax, casp3, and casp9. The GC-MS analysis of the A. spinosissima extract showed that 102 bioactive phytochemical compounds, which be of use to the synthesis of AgNPs. In conclusion, the results of this study indicate that phyto-synthesized AgNPs can be used to develop new therapeutic agents for cancer therapy.
Acknowledgement :
The authors would like to thank Mrs Dameshghian for her support in the present research.
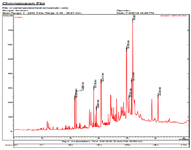
Figure 1. GC/MS chromatogram of A. spinosissima extract.
|
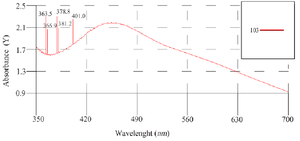
Figure 2. Ultraviolet-visible absorption spectra of phyto-synthesized AgNPs.
|
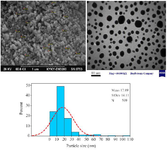
Figure 3. SEM and TEM images of phyto-synthesized AgNPs. Histogram of the particle sizes from TEM images.
|
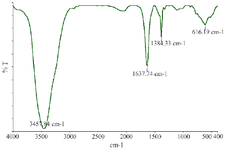
Figure 4. Fourier-transform infrared spectrum of phyto-synthesized AgNPs.
|
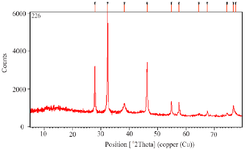
Figure 5. XRD analysis of phyto-synthesized AgNPs using A. spinosissima extract.
|
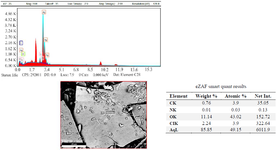
Figure 6. Energy-dispersive X-ray (EDX) spectrum of green synthesized AgNPs. EDX spectroscopy study was employed to detect the existence of elemental silver.
|
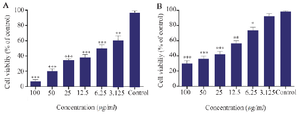
Figure 7. Cytotoxicity of phyto-synthesized AgNPs against MCF-7 (A) and L-929 (B) cell lines. (n=3: p<0.001***, p<0.01 **, p<0.05 *).
|

Figure 8. A) Amplification plot of casp3, casp9, Bax and Bcl2 apoptosis related genes, B) melting curve of mention genes.
|
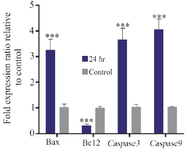
Figure 9. Apoptosis related genes expression analysis in MCF-7 cells treated with IC50 value of phyto-synthesized AgNPs (n=3: p<0.05 *, p<0.01 **, p<0.001***).
|

Figure 10. The flow-cytometric analysis of MCF-7 cells treated with phyto-synthesized AgNPs. The bottom left square: live cells, top left square: early apoptosis, bottom right square: necrosis, upper right square: delayed apoptosis.
|

Table 1. Chemical composition of A. spinosissima extract
|

Contd. Table 1. Chemical composition of A. spinosissima extract
|
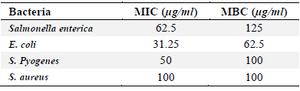
Table 2. Antibacterial activity of Phyto-synthesized AgNPs against pathogenic bacteria
|
|