Genome Analysis of an Enterococcal Prophage, Entfac.MY
-
Yazdanizad, Maryam
-
Department of Medical Biotechnology, Faculty of Advanced Science and Technology, Tehran Medical Sciences, Islamic Azad University, Tehran, Iran
-
Mazaheri Nezhad Fard, Ramin
-
Department of Pathobiology, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran 3. Food Microbiology Research Center, Tehran University of Medical Sciences, Tehran, Iran
-
Food Microbiology Research Center, Tehran University of Medical Sciences, Tehran, Iran
-
Salehi, Mohammadreza
-
Department of Infectious Diseases, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran
-
Dumanloo, Mahsa
-
Department of Clinical Pathobiology, Imam Khomeini Hospital Complex, Tehran University of Medical Sciences, Tehran, Iran
-
 Saboor Yaraghi, Ali Akbar
Department of Pathobiology, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran, asaboor@tums.ac.ir
Saboor Yaraghi, Ali Akbar
Department of Pathobiology, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran, asaboor@tums.ac.ir
-
Department of Pathobiology, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran 3. Food Microbiology Research Center, Tehran University of Medical Sciences, Tehran, Iran
Abstract: Background: Bacteriophages are bacterial parasites. Unlike lytic bacteriophages, lysogenic bacteriophages do not multiply immediately after entering the host cells and may integrate their genomes into the bacterial genomes as prophages. Prophages can include various phenotypic and genotypic effects on the host bacteria. Enterococcus spp. are Gram-positive bacteria that cause infections in humans and animals. In recent decades, these bacteria have become resistant to various antimicrobials, including vancomycin. The aim of this study was to analyze genome of an enterococcal prophage.
Methods: In this study, Enterococcus faecium EntfacYE was isolated from biological samples and its genome was analyzed using next-generation sequencing method.
Results: Overall, 254 prophage genes were identified in the bacterial genome. The prophage included 39 housekeeping, 41 replication and regulation, 80 structural and packaging, and 48 lysis genes. Moreover, 46 genes with unknown functions were identified. All genes were annotated in DNA Data Bank of Japan.
Conclusion: In general, most prophage genes were linked to packaging and structure (31.5%) gene group. However, genes with unknown functions included a high proportion (18.11%), which indicated necessity of further analyses. Genomic analysis of the prophages can be effective in better understanding of their roles in development of bacterial resistance to antibiotics. Moreover, identification and study of prophages can help researchers develop genetic engineering tools and novel infection therapies.
Introduction :
Enterococci are Gram-positive bacteria found in soil, water, plants and dairy products. Furthermore, they are naturally found in regular gastrointestinal microbiota of the vertebrates. These bacteria may be detected on the skin and mucosa as well as in the mouth and vagina 1,2. In addition, enterococci are commonly found in wastewater or fecal contaminated water 3. Of the enterococcal species, Enterococcus faecalis (E. faecalis) and Enterococcus faecium (E. faecium) majorly infect humans 4. Nowadays, antimicrobial resistance of E. faecium is a serious health threat according to the World Health Organization (WHO) and the US Centers for Disease Control and Prevention (CDC). Enterococci increasingly show resistance to various antimicrobial drugs and disinfectants, including important antibiotic of vancomycin. This resistance prolongs the hospital stay and increases overall medical costs as well as risks of secondary infections and mortalities in patients 2,5. Despite widespread phages in nature and their critical roles in medicine and industries, current knowledge of the biodiversity of these viruses is surprisingly small 4.
Bacteriophages generally include two infection cycles of lytic and lysogenic cycles. In lytic cycle, phages use host replication machinery to reproduce and eventually lyse the bacterial cells. In lysogenic cycle, phages integrate their genomes to the host genomes as prophages. Prophages may be conserved in the bacterial genomes for several years. Lysogenic phages enter the lytic phase (conversion) only when they are induced by natural factors (e.g. high temperatures) or laboratory agents (e.g. mitomycin C, hydrogen peroxide and UV radiation). In fact, conversion frequency of the prophages by natural inducers is low 6. In general, study on the phages can help development of genetic engineering tools and novel infection therapies. Therefore, the major aim of the present study was to genetically analyze Entfac.MY prophage in a clinical isolate of E. faecium.
Materials and Methods :
Bacterial strain
E. faecium was isolated from the biological sample of a hospitalized patient in Tehran, Iran, using routine microbiological methods. Bacterial isolation was verified using phenotypic methods such as Gram staining and biochemical methods such as catalase, oxidase, arabinose, bile esculin, NaCl, and PYR hydrolysis tests 7. Additionally, the bacterial strain was genetically verified via amplification of the enterococcal tuf gene using PCR technique and specific primers of forward Ent1: 5'-TACTGACAAACCATTCATGATG-3' and reverse Ent2: 5'-AACTTCGTCACCAACGCGAAC-3' 8. The PCR cycling conditions were as follows: initial denaturation for 5 min at 95°C, followed by 30 cycles of denaturation at 95°C for 30 s, annealing at 54°C for 30 s and extension at 72°C for 45 s. Final extension was carried out at 72°C for 7 min (modified from Mazaheri Nezhad Fard et al, 2010). The PCR product was sequenced using Sanger method (Kowsar Biotech, Iran). Furthermore, antimicrobial susceptibility assessment of the isolate was carried out using disk diffusion method for vancomycin, erythromycin, clinda-mycin, ceftriaxone, and cefoxitin antibiotics. Characterized enterococcal strain was then used to isolate bacteriophages.
Complete genome sequencing
In a previous study by Elahi et al, wastewater samples were used as the bacteriophage sources. After mixing 360 μl of each wastewater sample with 160 μl of the bacteria, suspension was poured onto a plate using two-layer agar method. Incubation was carried out at 37°C for 24 hr. After phage isolation on E. faecium strains, these bacterial strains were used for genome extraction 9. A small quantity of agar (containing bacteria and phages) was dissolved in 1 ml of Saline Magnesium (SM) buffer. After 10 min of centrifugation at 4480 g, supernatant was separated and used for the bacterial genome extraction. Genome extraction was carried out using High Pure Viral Nucleic Acid Kit (Roche, Switzerland) as follows: 200 μl of working solution were added to a sterile microtube and mixed with 50 μl of proteinase K. After addition of supernatant to the microtube, solution was mixed well using vortex and incubated at 72°C for 10 min. Then, 100 μl of binding buffer were added to the solution and mixed well. After transferring filter column into the microtube, 600 μl of the solution were added to the filter. Solution was centrifuged at 8000 g for 1 min at 4°C. Filter was transferred into a new microtube and 500 μl of inhibitor removal buffer were added to the filter. Solution was centrifuged at 8000 g for 1 min at 4°C. After transferring filter to a new microtube, 450 μl of wash buffer were added to the filter. Solution was centrifuged at 8000 g for 1 min at 4°C. Then, filter was transferred into a fresh microtube and the last two steps were repeated. To dry the filter, it was centrifuged at 13000 g for 10 s at 4°C. Filter was then transferred into a new sterile microtube and 30 μl of the elution buffer were added to the filter. After 1 min of setting at room temperature, centrifuge was carried out at 8000 g for 1 min at 4°C. The extracted bacterial genome was wholly sequenced using Illumina HiSeq Platform (Novogene, China) and analyzed using SPAdes de novo and reference assembly technologies. Technically, Illumina is a Next-Generation Sequencing (NGS) technology, which uses a proprietary reversible terminator-based technique that detects single bases as they are incorporated into the DNA template strands. In this study, prophage was analyzed using RAST online sequence analysis service and results were publicly deposited in DNA Data Bank of Japan (DDBJ).
Results :
Results of phenotypic and biochemical tests verified the primary characteristics of the enterococcal isolate. Gram-staining results showed that the isolate was a Gram-positive coccus. Results of the biochemical tests were reported as follows: negative catalase, negative oxidase, negative arabinose, positive bile esculin and positive PYR hydrolysis. Results of Sanger sequencing for the bacterial tuf gene also verified molecular characteristics of the enterococcal isolate (DDBJ accession numbers of LC580430 and LC580431). Antimicrobial susceptibility assessment results showed that E. faecium isolate was resistant to commonly used antibiotics, including vancomycin, erythromycin, clindamycin, ceftriaxone and cefoxitin. In general, Entfac.MY prophage included 130241 nucleotides with 35.74% A, 27.08% T, 16.02% C and 21.15% G. In total, 254 prophage genes were analyzed and grouped based on their functions (Table 1). These included 39 housekeeping genes, 41 replication and regulation genes, 80 packaging and structural genes, and 48 lysis group genes. Moreover, 46 genes with unknown functions were analyzed (Figure 1).
Discussion :
In general, bacteriophages isolated by exposing a clinical strain of E. faecium to wastewater samples included lysogenic phages. After complete genomic sequencing of Entfac.MY prophage, 254 genes were functionally analyzed. The housekeeping group of genes included YhgE/Pip domain-containing protein (12 copies), CHAP domain-containing protein, phage tail protein (ten copies), RloB domain-containing protein, antireceptor, LysM peptidoglycan-binding domain-containing protein, ORF6C domain-containing protein, VRR-NUC domain-containing protein, Ig domain-containing protein (two copies), portal protein (ten copies), AAA family ATPase (four copies), phage tail family protein (two copies) and phage head-tail connector protein. Portal protein forms a channel for two-way passes of the viral DNA. The EFRM31 phage genome also included this gene 10. The functional mechanisms of AAA family ATPase, including cell cycle regulation, proteolysis and protein breakdown and intracellular transport, were investigated in 2002 11.
The phage tail family protein encodes tail components. The antireceptor gene is involved in identifying and binding phage to its bacterial hosts. Function of this gene was previously reported by Vegge et al 12 and Duplessis and Moineau 13. The phage head-tail connector protein gene is involved in the assembly of virions by connecting their heads and tails.
The replication and regulation group of genes included IS607 family transposase, ribbon-helix-helix domain-containing protein, recombinase RecT (three copies), phage regulatory protein, thermonuclease family protein, helix-turn-helix transcriptional regulator (three copies), MazG-like family protein (two copies), DNA polymerase (two copies), DNA primase, deoxyribonuclease IV (five copies), recombinase family protein (four copies), DEAD/DEAH box helicase family protein, phage antirepressor, helix-turn-helix domain-containing protein (three copies), peptidoglycan recognition protein, nucleoid-associated protein (three copies), DNA methyltransferase and phage holin (six copies). In fact, DNA polymerase gene produces enzymes that act in pairs to produce two identical DNA strands from an original DNA molecule and are essential for DNA replication. Krzywkowski et al studied this gene in 2018 14. Naturally, DNA primase is an enzyme involved in DNA replication and is linked to RNA polymerases. Structure and mechanism of this gene have been studied by O’Brien et al in 2018 15 and Lee et al in 2010 16. When cells are in repairing process of the nucleotide cleavage pathway, deoxyribonuclease IV is active in aqueous or aporin-apyrimidinic sites. The gene function has been explained by Enriquez et al 17. Recombinase family protein produces enzymes involved in repairing DNA damages. Voziyanova et al investigated function and evolution of this gene 18. The DEAD/DEAD box helicase family protein produces enzymes that bind and hydrolyze NTP to double-stranded (ds) RNA molecules or regenerate RNA protein complexes. The phage antirepressor gene prevents suppressor proteins from binding to their operators. This mechanism has recently been investigated by Silpe et al in 2020 19. The helix-turn-helix domain-containing protein can bind to DNA molecules. The peptidoglycan recognition protein gene detects peptidoglycan in the bacterial cell walls; as described by Skerry et al 20 and Jiang et al 21. Nucleoid-associated proteins naturally include abundant polypeptides with Low-Molecular Weights (LMW) that bind DNA molecules and change their shapes and abilities to participate in processes such as transcription. Sometimes, these proteins can bind RNA molecules and post-transcriptionally affect the gene expression of cells; as described by Stojkova et al in 2019 22. DNA methyltransferases are a family of enzymes involved in production and maintenance of CpG methylation in the genome; as described by Furuta et al in 2014 23 and Stoddard et al in 2019 24. Holins include diverse groups of small proteins from dsDNA phages that destruct the host cell walls in the virus lytic cycle. Functional mechanisms of the holins were previously described by Bardina et al 25, Stamereilers et al 26 and Jacobs et al 27.
The packaging and structure group of genes included tail tape measure protein (24 copies), P27 family phage terminase small subunit, baseplate J/gp47 family protein (two copies), PBSX family phage, phage gp6-like head-tail connector protein (two copies), collagen-like protein, phage major tail protein, BppU family phage baseplate upper protein, HK97 family phage prohead protease (three copies), phage scaffolding protein, terminase large subunit (17 copies), phage major capsid protein (11 copies), minor capsid protein, phage baseplate upper protein, structural protein (three copies) and small subunit of terminase (two copies). The terminase large subunit gene is involved in viral DNA transfer and packaging termination. The phage major capsid protein gene encodes capsid proteins and was previously reported in the phage genome of EFRM31 in 2010 10. Stamereilers et al reported this gene as well 26. Another gene involved in encoding capsid proteins is the minor capsid protein gene. In 2015, Pawlowski et al studied functions of this gene in P23-77 phage 28. The phage baseplate upper protein gene was also reported by Li et al in ϕ11 genome 29. Structural protein gene plays an important role in shaping structure of the virus. In 2015, McNulty et al studied terminase small subunit gene, which is responsible for binding of the encoded protein to several identifying elements at the beginning of viral packaging 30. In addition, Roy et al studied this gene in 2011 31.
The lysis group of genes included hemolysin XhlA family protein (three copies), 1,4-β-N-acetylmurami-dase, XkdX family protein (three copies), site-specific integrase (21 copies), N-acetylmuramoyl-L-alanine amidase (three copies), lysin, endonuclease, tyrosine-type recombinase/integrase (six copies), peptidoglycan endopeptidase EnpA (three copies), autolysin, HNH endonuclease (four copies) and lysozyme family protein. Site-specific integrase gene is responsible for rearranging DNA fragments; as previously identified by Petersen et al in the genome of TPW22 phage 32. This gene was also identified in TP901-1 phage genome 33. The N-acetylmuramoyl-L-alanine amidase gene produces an enzyme that catalyzes a chemical reaction and cleaves the link between N-acetylmuramoyl and L-amino acid residues in the cell-wall glycopeptides. Bierbaum et al have described functional mechanisms of this gene 34. Lysines are hydrolytic phage enzymes produced to separate the host cell walls in lytic cycles. In 2015, LeBlanc et al reported this gene in their phage genome 35. The endonuclease gene produces enzymes that break down DNA molecules at specific locations. The tyrosine-type recombinase/integrase gene is involved in DNA binding and recombination. In a similar study in 2021, Malecki et al reported peptidoglycan endopeptidase EnpA gene in an enterococcal prophage 36. The autolysin gene is an enzyme that breaks down the components of peptidoglycans in cells and separates daughter cells after cell division. Ju et al in 2012 37 and Kohler et al in 2014 studied this gene 38. The HNH endonuclease gene plays various roles in the phage life cycle as the major component of the phage DNA packaging machinery. In 2017, Zhang et al studied the mechanism of this gene in GVE2 phage genome 39. Lysozyme is a glycoside hydrolase that catalyzes the hydrolysis of 1,4-β bonds between N-acetylmuramic and N-acetyl-D-glucosamine residues of the peptidoglycan. Previously, Irwin studied this gene in 2014 40.
The unknown function group of genes included PcfJ family protein, DUF2800 domain-containing protein, DUF4145 domain-containing protein, DUF2815 family protein, siphovirus Gp157 family protein, toxin (two copies), DUF1073 domain-containing protein, siphovirus ReqiPepy6 Gp37-like family protein, DUF2213 domain-containing protein, DUF3383 family protein, DUF4065 domain-containing protein, DUF2184 domain-containing protein (two copies) and hypothetical protein (31 copies). Hypothetical proteins were previously addressed by Mazaheri et al in EFRM31 10 and Tang et al in ϕNJ2 41. Based on the complete genomic analysis of an enterococcal phage by Mazaheri Nezhad Fard et al in 2010, genes similar to the genes of this study were reported, including hypothetical proteins, portal proteins, major capsid proteins, and holins 10. In another study by Tan et al in 2007, genes similar to the genes of the current study were reported as well, including terminase large subunit and portal protein genes 42. In a study by O'Flaherty et al on staphylococcal phage genomes (2004), 63 hypothetical protein genes were identified 43 while 31 hypothetical protein genes were identified in the present study. In the present study, AAA family ATPase, holin, and major capsid protein genes were successfully characterized.
Conclusion :
In general, bacteriophages are present in all environments with alive bacteria, especially in wastewaters. Bacteriophages can insert their genomes into the bacterial genomes to become prophages. Prophages can regulate bacterial populations by changing the bacterial gene expression rates and are involved in bacterial resistance by transferring antibiotic resistance genes to their bacterial hosts. Up-to-date, pathogenic bacteria have developed multiple resistance to available antibiotics, creating serious problems with costly infection treatments and unwanted mortalities. Bacteriophages can be used as novel solutions for these problems. Therefore, genomic analysis of the prophages can improve better understanding of their effects on bacteria. Furthermore, study of prophages can help researchers develop novel genetic engineering tools and effective medical therapies.
Acknowledgement :
The authors thank staff within the Microbiology Laboratory, Department of Pathobiology, School of Public Health, Tehran University of Medical Sciences.
Conflict of Interest :
The authors declare no conflict of interest.
Ethics Statement :
The current study was carried out based on the methods approved by the Ethics Committee of Tehran University of Medical Sciences (IR.TUMS.SPH.REC. 1397.139).
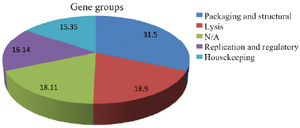
Figure 1. Major gene groups of Enfac.MY prophage.
|

Table 1. Genomic analysis of the Enfac.MY prophage and its similarity to other analyses from GenBank
|

Contd. Table 1. Genomic analysis of the Enfac.MY prophage and its similarity to other analyses from GenBank
|

Contd. Table 1. Genomic analysis of the Enfac.MY prophage and its similarity to other analyses from GenBank
|

Contd. Table 1. Genomic analysis of the Enfac.MY prophage and its similarity to other analyses from GenBank
CDS: Coding Sequence, bp: Base Pair, DDBJ: DNA Data Bank of Japan, GenBank: GenBank Protein ID for this study, Cv: Coverage, Id: Identity, N/A: Not Applicable or Announced, L: Lysis, PS: Packaging and structural, H: Housekeeping, RR: Replication and regulation.
|
|