Antitumor Activities of Green Tea by Up-regulation of miR -181a Expression in LNCaP Cells Using 3D Cell Culture Model
-
 Safari, Fatemeh
Department of Biology, Faculty of Science, University of Guilan, Rasht, Iran, Tel: +98 133 3333647; E-mail: fsafari@guilan.ac.ir
Safari, Fatemeh
Department of Biology, Faculty of Science, University of Guilan, Rasht, Iran, Tel: +98 133 3333647; E-mail: fsafari@guilan.ac.ir
-
Rayat Azad, Narjes
-
Department of Biology, Faculty of Science, University of Guilan, Rasht, Iran
-
Alizadeh Ezdiny , Ali
-
Department of Biology, Faculty of Basic Sciences, Rasht Branch, Islamic Azad University, Rasht, Iran
-
Pakizehkar, Safoora
-
Cellular and Molecular Endocrine Research Center (CMERC), Research Institute for Endocrine Science, Shahid Beheshti University of Medical Sciences, Tehran, Iran
-
Khazaei Koohpar, Zeinab
-
Department of Cell and Molecular Biology, Faculty of Biological Sciences, Tonekabon Branch, Islamic Azad University, Tonekabon, Iran
-
Ranji, Najmeh
-
Department of Biology, Faculty of Basic Sciences, Rasht Branch, Islamic Azad University, Rasht, Iran
Abstract: Background: Prostate Cancer (PCa) is the major reason for the high mortality rates among men worldwide. In fact, current therapeutic approaches are not successful. It appears that discovering more effective methods considering several parameters such as availability, low cost, and no toxicity to normal cells is one of the biggest challenges for interested researchers. Green tea (extracted from the plant Camellia sinensis) with high level of polyphenolic compounds and as the most globally consumed beverage has attracted considerable interest. MicroRNAs (or miRNAs) were considered as novel tools in cancer therapy which modulate various biological events in cell by regulation of gene expression. The aim of the current study was to evaluate the antitumor activity of green tea in LNCaP cells through up-regulation of miR-181a expression.
Methods: First, LNCaP cells were cultured and by using quantitative real time PCR (qRT-PCR) and western blot methods, the expression levels of Bax and BCL2 were analyzed. Next, a 3D cell culture model was applied to evaluate the expression of miRNA-181a in LNCaP cells.
Results: It was shown that green tea induced cellular apoptosis. The high number of apoptotic nuclei was also shown by using DAPI staining. The inhibition of tumor growth was revealed by analyzing the size and number of spheroids. Also, up-regulation of miR-181a expression in LNCaP cells was revealed after treatment with green tea.
Conclusion: Our results are helpful to design antitumor regimens based on consumption of green tea through up-regulation of miRNA-181a expression and induction of apoptosis.
Introduction :
Cancer is a multifactorial disease with high death rates in human. Among different cancer types, Prostate Cancer (PCa) is one of the leading causes of death among men worldwide 1. Hormone therapy, surgery, radiation, and chemotherapy are current approaches for treatment of PCa patients. Resistance to anticancer drugs and their side effects are well recognized as major reasons for the failure of cancer treatment and therefore, the identification of new tools and specific platforms with the lowest side effects especially from natural products is urgently needed. In herbal therapy, it seems that plants may serve as potent cost effective chemotherapeutic agents with less toxicity to normal mammalian tissues 2. In this regard, protective effects of green tea as an herbal medicinal product have attracted considerable interest. Green tea is one of the most popular beverages worldwide which is produced from the leaves of the Camellia sinensis plant. Green tea contains a group of polyphenolic compounds including four main catechins of (−)-epicatechin (EC), (−)-epigallocatechin (EGC), (−)-epicatechin-3-gallate (ECG), and (−)-epigallocatechin-3-gallate (EGCG) 3,4. Healthy effects of green tea on many human diseases have been documented 5-8. Moreover, the antitumor activities of green tea on different types of human cancers have been discovered 9-12.
MicroRNAs (miRNAs) are new promising targets in cancer therapy. miRNAs are single-stranded non-coding RNAs involved in various cellular pathways, negatively or positively regulating their target genes 13,14. Therefore, by using miRNAs, new therapeutic approaches and diagnostic tools to treat cancer were employed 15. In PCa, the implication of several miRNAs in modulating a variety of biological processes including proliferation, migration, invasion, and apoptosis was shown 16-19. miR-181a belongs to the miR-181 family. It was reported that miR-181a can act as an oncogenic miRNA or tumor suppressor miRNA and thereby, the exact role of miR-181a in tumorigenesis is not clearly understood 20-27. It seems that the role of miR‑181a in tumorigenesis is tumor-specific. On the other hand, several reports also showed the inhibitory effects of purified green tea catechins on the cancer cells by the dysregulation of miRNAs 28-31.
In this study, an attempt was made to study the anticancer effects of green tea against LNCaP human prostate cancer cells through changing miR-181a expression in 3D cell culture model. By using DAPI staining, quantitative real-time PCR (qRT-PCR) and western blot, the induction of apoptosis was shown. Moreover, the antitumor effects of green tea on LNCaP cells through miR-181a were evaluated in 3D cell culture model. Based on our results, it was found that green tea may be a potential candidate and a novel natural product in the treatment of prostate cancer patients through up-regulation of miR-181a expression.
Materials and Methods :
Sample preparation and extraction: The fresh leaves of related plants were collected from Lahijan city, Guilan province, Iran in March 2018. It was considered as Camellia sinensis in the Department of Pharmacognosy at Tehran University of Medical Sciences and the herbarium was registered as THE-6561. Plant extraction was performed as previously described 12. Briefly, the fresh leaves were dried at room temperature and darkness for two weeks, and the powder was obtained. The powder was soaked in 50% pure ethanol (Merck, Germany) +50% water for three days. The extract was filtered and stored at -20°C.
Cell line and culture: Lymph node carcinoma of the prostate (LNCaP) was provided from Pasture Institute (Tehran, Iran), grown in DMEM medium, and supplemented with 10% Fetal Bovine Serum (FBS; Bioidea BI201, Iran), 100 μg/ml penicillin G/streptomycin and 1% L-glutamine.
MTT assay: The effect of green tea extract on the viability of LNCaP cells was measured through 3-(4,5-dimethy-lthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay (CatNo:BI1017, Bioidea, Iran) based on the ability of live cells to cleave the tetrazolium ring into a molecule by absorbance at 490 nm based on manufacturer’s instructions. For this purpose, LNCaP cells were plated in 96-well culture plates (5×103 cells/well), incubated for 24 hr, and treated with green tea extract. After 48 hr at 37°C, the media were removed, 100 μl of MTT reagent (1 mg/ml) was added into each well, and the cells were incubated at 37°C for 4 hr. Next, the MTT solution was removed and 50 μl of dimethyl sulfoxide (DMSO) was added into each well to dissolve formazan crystals. Finally, the plates were gently shaken for 10 min and read with an ELISA plate reader (BioTek, USA) 12. In this study, etoposide and deionized water were used as positive and negative controls, respectively.
DAPI staining assay: DAPI (4,6-Diamidino-2-phenylindole dihydrochloride) staining assay was used to determine the changes in the chromatin of LNCaP cells after treatment with green tea extract for 24 hr. DAPI was purchased from Sigma-Aldrich ,USA. Briefly, LNCaP cells were seeded in the six-well plates (5×104 cells per well) containing 12 mm cover slips, and consequently treated with green tea extract for 24 hr. Then, the cells were fixed with 3.7% paraformaldehyde, permeabilized in 0.5% (w/v) Triton X-100 and 1% BSA (w/v) for 5 min, washed in Phosphate-Buffered Saline (PBS), and stained through DAPI. All images were taken by using Eclipse Ti-E inverted fluorescent microscope (Nikon, Japan) 12.
Antibodies, SDS-PAGE, and western blot: Anti-β-Actin Antibody (C4) (Santa Cruz Biotechnology, USA), Anti-Bax Antibody (B-9) (Santa Cruz Biotechnology, USA), Bcl-2 Antibody (N-19) (Santa Cruz Biotechnology, USA) were used as primary antibodies for immunoblotting and western blot was performed as previously described 32.
RNA and miRNA extraction, cDNA synthesis, and quantitative real-time PCR (qRT-PCR): In order to perform quantitative real-time RT-PCR analysis, LNCaP cells were lysed after 48 hr along with culturing green tea extract. In addition, total RNA was extracted by using 500 μl of Trizol® reagent based on the manufacturer-provided protocol (Invitrogen Life Technologies, USA), followed by reverse transcription into cDNA by considering manufacture's protocol (ReveretAid M-Mulv reverse transcriptase kit, Thermo Fisher Scientific, USA). Further, real-time RT-PCR was implemented to amplify cDNA using SYBR Green dye universal master mix (Bioron GmbH, Germany) in Light Cycler 480 (Roche Diagnostics, USA) using the primers for GAPDH-F: 5′-CAA GGT CAT CCA TGA CAA CTTTG-3′, R:5′-GTCCACCACCCTGTTGCTG TAG-3′, Bax-F: 5′-GTCGCCCTTTTCTACTTTGCC-3′, R: 5′-CTCCCGCCACAAAGATGGTCA-3′, BCL-2F: 5′-CCCCTCGTCCAAGAATGCAA-3′, and R: 5′- TCTCCCGGTTATCGTACCCTG-3′ for forty cycles. Data represent average copy number normalized to the GAPDH housekeeping gene. Also, the primers were synthesized by Pishgam Biotech Co. in Iran. A reaction similar to the above but with deionized water instead of cDNA was set as a negative control. Thermal conditions of the PCR consisted of primary denaturation at 94°C for 2 min, 45 cycles of denaturation at 94°C for 30 s, annealing at 59°C for 30 s, and amplification at 72°C for 30 s 12 (miR-181a-F: 5′-ACCAACATTCAA CGCTGTC-3′; universal reverse primer: 5′-GAGCA GGGTCCGAGGT-3′). miR-181a and universal reverse primers were purchased from Bon Yakhteh in Iran. The delta Ct values of the samples were classified, and the expression levels were studied using 2-ΔΔCt (Fold change) values.
Hanging drop formation: The hanging drop method was performed to create a 3D cell culture model and form spheroid 33. In this regard, LNCaP cells were cultured when they had 90% confluence, trypsinized and counted. In addition, ten 20-µl drops involving 20×103 cells were pipetted into the lid of tissue culture dish (60 mm) and 5 ml of PBS was placed in its bottom. The experiment was conducted for the control (cells+medium) and sample (cells+ medium containing green tea extract). Furthermore, the number and size of spheroids was determined after three days. Briefly, spheroid formation was monitored using a phase contrast inverted microscope (INV100, BEL Engineering, Italy) and spheroid number was calculated 32.
Statistical analysis: The data were analyzed by SPSS v22 (Chicago, USA) and graphs were drawn using Graphpad Prism 7 software. Additionally, the data are expressed as means±standard deviation (SD). Further, the experiments were performed three times and two-tailed Student’s t test was used to compare the two groups. Finally, p-value less than 0.05 was considered statistically significant 12.
Results :
The role of green tea in inducing apoptosis in LNCaP cells: The anti-apoptotic activities of green tea against LNCaP cells were investigated by using MTT assay. To do so, LNCaP cell line was cultured and then treated with different concentrations of green tea extract (100-1000 µg/ml) for 48 hr (Figure 1A). Next, the morphology of LNCaP cells was analyzed before and after treatment with green tea extract (Figure 1B). To further determine the effect of green tea extract on apoptotic cell death, the cellular nuclear morphology changes of LNCaP cell line were examined by DAPI staining and nuclear fragmentation was clearly shown (Figure 1C). The number of apoptotic nuclei in the cells of the control and sample was compared (Figure 1D). By using quantitative real-time PCR and western blot, the expression of Bax and BCL2 was finally evaluated (Figures 1E, 1F, and 1G). Based on the results, it was found that green tea induces the cellular apoptosis in LNCaP cells.
Antitumor effects of green tea on LNCaP cells via up regulation of miR-181a using 3D cell culture model: A 3D cell culture system is a good reflection of the in vivo cell behaviors. By using hanging drop method, spheroids are formed in droplets 33. As explained in Materials and Methods section, LNCaP cells were cultured, trypsinized, and counted. Ten drops containing 20×103 cells were pipetted into the bottom of the tissue culture dish lid and PBS was placed in its bottom (Figure 2A). After spheroid formation (around 3 days), the expression of Bax and BCL2 were evaluated (Figures 2B and 2C). Next, the size and number of spheroids were analyzed which represent the smaller size and number in the sample (Cells+medium containing green tea) compared to the control (Cells+medium) (Figures 2D and 2E). Finally, the expression of miR-181a in the sample and control group was evaluated. To do so, two days after spheroid formation, LNCaP cells were harvested and the expression of miR-181a was evaluated by qRT-PCR method (Figure 2F). Taken together, these results suggested that green tea has therapeutic effects on LNCaP cells using 3D cell culture model and miR-181a expression was elevated in LNCaP cancer cells after green tea treatment.
Discussion :
PCa is considered as one of the most important causes of cancer-associated death in men worldwide. PCa therapy remains a challenge for interested researchers. Moreover, correlation between dietary factors and diseases has been reported. Interestingly, miRNAs have been reported to be potential biomarkers for diagnosis and treatment of cancers due to their management of cellular dysregulations and deregulated expression in a variety of human cancer types. In the present study, the purpose was to evaluate the in vitro antitumor activities of green tea in LNCaP cells. In this regard, 2D and 3D cell culture models were employed for performing our experiments. It was found that green tea inhibited the growth and induced apoptosis in LNCaP cells. Also, it was shown that miR-181a was up-regulated in LNCaP cells after treatment with green tea using 3D cell culture model. Consistent with our finding that miR-181a may act as a tumor suppressor, Shen et al showed that the overexpression of miR-181a could significantly inhibit cell proliferation and induce G1 cell cycle arrest in PCa 34. Also, Huang et al. showed that the overexpression of miRNAs members suppressed Non-Small Cell Lung Cancer (NSCLC) proliferation, migration, and invasion and induced cellular apoptosis through BCL2 targeting 35. In Glioblastoma, it was found that miR-181a acts as a tumor suppressor by inhibition of cell growth, invasion, and by induction of cell apoptosis 26. In another study, it was found that overexpression of miR-181a in K562CML cells inhibits cell growth and induces cellular apoptosis and differentiation. Moreover, it was revealed that overexpression of miR-181a enhances the chemotherapeutic sensitivity of K562 CML cells to imatinib 36. In cutaneous squamous cell carcinoma, miR-181a reduces cell viability and suppresses cell apoptosis via down-regulating KRAS 37. In breast cancer, it was demonstrated that miR-181a-5p inhibits migration and angiogenesis of cancer cells through down-regulation of matrix metalloproteinase-14 38.
In contrast with tumor suppressor roles of miR-181a, the oncogenic roles of miR-181a were shown by several studies. In this regard, it was found that miR-181a enhanced cell viability and diminished apoptosis by targeting Kruppel-Like Factor 6 (KLF6) in clear renal cell carcinoma 39. Tong et al reported that miR-181a was up-regulated in prostate cancer tissues compared with adjacent normal tissues 19. Also, miR-181a activates the Epithelial-Mesenchymal Transition (EMT) process in prostate cancer cells through inhibition of TGIF2 (A repressor of the Smad pathway) 40. Also, the expression level of plasma miR-181 in pancreatic cancer patients was elevated 41 and it was shown that miR-181a promotes pancreatic cancer invasion and progression via targeting the tumor suppressor genes, phosphatase and tensin homolog (PTEN) and mitogen-activated protein kinase kinase 4 (MAP2K4) 42. Oncogenic role of miR-181a through suppression of WIF-1 (Wnt inhibitory factor-1) in colorectal carcinoma was also shown 43. It seems that tumor suppressor or oncogenic roles of miR-181a may be dependent on the cellular and tumor context.
Conclusion :
In the present study, it was found that green tea has therapeutic influences on LNCaP cells by induction of apoptosis, suppression of tumor growth, and up regulation of miR-181a expression. However, the targeting of miR-181a in LNCaP cells treated with green tea is unknown. miRNAs play a broad range of roles in cellular processes and they are currently considered as a promising tool in cancer therapy. Therefore, more studies are required to identify the exact molecular mechanisms of miR-181a in prostate cancer after treatment with green tea.
Conflict of Interest :
The authors declare that there is no conflict of interest to disclose.
Funding Statement :
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
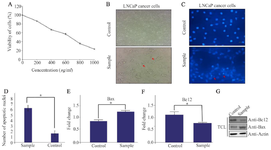
Figure 1. A) The effects of green tea on LNCaP cell viability in different concentrations using MTT assay. B) Comparison of morphological changes of LNCaP before and after treatment with green tea. The experiments were performed three times (original microscope magnification, 20X, scale bar, 10 μm). C, D) DAPI staining of LNCaP cells under treatment with green tea (sample) compared with control. The experiments were performed three times (original microscope magnification, 40X, scale bar, 10 μm). E, F) Relative fold-changes in gene expression levels of Bax and BCL2 were shown. Data represent mean±SD of three independent experiments. * p<0.05 was considered statistically significant. G) The expression of Bax and BCL2 proteins using western blot was shown. Actin used as an internal control (TCL: Total Cell Lysate).
|
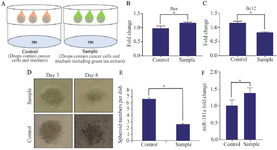
Figure 2. A) Schematic model of spheroid formation using hanging drop technique. B, C) Relative fold-changes in gene expression levels of Bax and BCL2 in LNCaP cells after treatment with green tea (five days) were shown. Data represent mean±SD of three independent experiments. *p<0.05 was considered statistically significant. D, E) Medium containing green tea inhibits size and number of spheroids (magnification: 20x, scale bar: 100 μm; three independent experiments were done. * p<0.05 was considered statistically significant). F) Relative fold-changes in gene expression levels of miR-181a in LNCaP cells during treatment with green tea were shown. Data represent mean±SD of three independent experiments. *p<0.05 was considered statistically significant.
|
|