Synchronous Comparison of Mycobacterium tuberculosis Epidemiology Strains by "MIRU-VNTR" and "MIRU-VNTR and Spoligotyping" Technique
-
 Jafarian, Mehdi
Mehdi Jafarian, M.Sc., Mycobacterium Research Center (MRC), National Research Institute of Tuberculosis and Lung Disease (NRITLD), Shahid Beheshti University of Medical Sciences, Tehran, Iran, Tel: +98 21 20109505 - E-mail:mehdijafariandormency@gmail.com
Jafarian, Mehdi
Mehdi Jafarian, M.Sc., Mycobacterium Research Center (MRC), National Research Institute of Tuberculosis and Lung Disease (NRITLD), Shahid Beheshti University of Medical Sciences, Tehran, Iran, Tel: +98 21 20109505 - E-mail:mehdijafariandormency@gmail.com
-
Mycobacteriology Research Center, National Research Institute of Tuberculosis and Lung Disease (NRITLD), Shahid Beheshti University of Medical Sciences , Tehran, Iran
-
Aghali-Merza, Muayed
-
Mycobacteriology Research Center, National Research Institute of Tuberculosis and Lung Disease (NRITLD), Shahid Beheshti University of Medical Sciences , Tehran, Iran
-
Farnia, Parissa
-
Mycobacteriology Research Center, National Research Institute of Tuberculosis and Lung Disease (NRITLD), Shahid Beheshti University of Medical Sciences , Tehran, Iran
-
Ahmadi, Mojtaba
-
Mycobacteriology Research Center, National Research Institute of Tuberculosis and Lung Disease (NRITLD), Shahid Beheshti University of Medical Sciences , Tehran, Iran
-
Masjedi, Mohammad Reza
-
National Research Institute of Tuberculosis and Lung Disease (NRITLD), Shahid Beheshti University of Medical Sciences , Tehran, Iran
-
Velayati, Ali Akbar
-
National Research Institute of Tuberculosis and Lung Disease (NRITLD), Shahid Beheshti University of Medical Sciences , Tehran, Iran
Abstract: Molecular epidemiology analyses are frequently used in determining epidemiology of tuberculosis. Recently, Mycobacterial Interspersed Repetitive Unit Variable Number Tandem Repeat (MIRU-VNTR) and Spoligotyping has become an important method, as it allows high-through put, discriminatory and reproducible analysis of clinical isolate. The purpose of this study is to compare techniques of “MIRU-VNTR” versus “MIRU-VNTR and Spoligotyping” together for study of genetic pattern of Mycobacterium tuberculosis (M. tuberculosis) strains. Sixty M. tuberculosis (MTB) isolates were selected (30 susceptible, 30 multi-drug resistant) for this study. Thereafter, the "MIRU-VNTR and spoligotyping" were performed to identify their genetic patterns. The frequency of unknown genetic pattern of MTB was compared using technique of “MIRU-VNTR” alone versus “MIRU-VNTR and Spoligotyping” together. The MIRU-VNTR allelic diversity at each of the loci was calculated by Hunter – Gaston Discriminatory Index (HGDI). Based on differentiation index of all strains 10, 16, 26, 31 and 40 loci were identified as the most distinctive (HGI = 0.6) and 2, 4, 20 and 24 as the weakest distinctive locus (HGI = 0.3). By using MIRU-VNTR technique 38% (n= 23) of isolates could not be typed, whereas by applying "MIRU-VNTR and Spoligotyping" together only 15% (n= 9) of isolates remained unknown (p = 0.004). For sensitive strains, the difference was significant (67% vs. 90%, p = 0.028), but only marginally significant for drug resistant strains (57% vs. 80%, p = 0.052). The discrimination power of 12-locus MIRU-VNTR and Spoligotyping was equal to that of MIRU-VNTR analysis. If appropriate loci are added to the standard MIRU analysis, MIRU-VNTR genotyping could be a valuable tool for strain typing and epidemiological research of M. tuberculosis. With this approach a more clear understanding about genetic pattern of MTB can be achieved.
Introduction :
Tuberculosis is one of the main factors of vital statistic in the third world countries. Ninety percent of all tuberculosis patients live in third world countries (1). In some regions like Saharan desert in Africa 1000 of 100,000
people are infected with tuberculosis and HIV co-infection, among which one out of three dies from HIV virus (2). Recent, spread and rise in multi-drug resistant M. tuberculosis (MTB) isolate had worsened the matter. In addition, the worldwide development of transport and migration contributes to globalize these threats. Therefore, there is an urgent need to understand and estimate the incidence of tuberculosis in large and small biographical ranges (3).
In this context, effective methods for accurate identification and typing are required. However, all the current typing markers suffer from significant drawbacks. For example DNA fingerprinting method based on IS6110 is a powerful tool to study molecular epidemiology of MTB isolates. But RFLP based on IS6110 typing is difficult and time consuming, and cannot be applied to MTB strains with low or no IS6110 banding patterns (4).
Spoligotyping analyzes the Direct Repeat (DR) locus in the genome of M. tuberculosis, which is composed of a cluster of 36 bp repeat sequences interspersed with unique spacers of 35 to 41 bp. By using a reverse Southern blotting technique, the variability in the spacer sequences can be interrogated and recorded in a digital code (5). Although this method provides digital typing data, it is only measuring variability in a single locus and does not generally provide sufficient discrimination for outbreak investigation (6).
Another molecular technique for strain typing of MTB is based on Variable Number Tandem Repeats (VNTRs) of Mycobacterial Interspersed Repetitive Units (MIRUs). The repeated units are 52 to 77 nucleotides in length and the number of repeated units can be determined by the size of the entire locus. Previous studies demonstrated the importance of MIRU-VNTR method for tracking epidemiological key events such as transmission or relapse and provide non-ambiguous data which are highly portable between different laboratories (7).
The purpose of this study was to compare the frequency of unknown genetic pattern of MTB isolates by using single technique of "MIRU-VNTR" versus the combined techniques of "MIRU-VNTR and Spoligotyping".
Materials and Methods :
Design and setting
The study was conducted in Mycobacteriology Research Center, NRITLD, Shahid Beheshti University of Medical Sciences, Tehran, Iran (2009).
Participants and sampling
Sixty MTB isolate along with their clinical files (30 sensitive and 30 multi-drug resistant MTB from Mycobacteriology bank in MRC) were used for this study.
Extraction DNA
DNA from M. tuberculosis isolates was extracted from growth on LJ slants by a well standized method. In brief, fifty ?l of Lysozyme (Merck, Germany) was added to the mycobacterial suspension in 400 µl of water, followed by stirring and incubation at 37 ?C. Ten µl with 10 mg/ml of proteinase K (Merck, Germany) was added to the sample, which was incubated for 10 min at 65?C, and then heated at 100?C for 30 min. One volume of phenol/ chloroform/ isoamyl-alcohol 25: 24: 1 was added to the supernatant, mixed by inversion and centrifuged at 12,000 xg for
10 min. The aqueous phase was transferred to other tubs, and the extraction procedure was repeated. A volume of chloroform/ isoamyl- alcohol 24: 1 was added and mixed by inversion followed by centrifugation for 10 min at 12,000 xg. One hundred µl of 5 M Nacl and two volumes of absolute ethanol were added to the supernatant. Samples were incubated for 60 min at -20?C and centrifuged for 15 min at 12,000 xg. The pellet was washed twice with 1 ml of 70% ethanol and re suspended in 200 µl of 0.1 x TE buffer (1 mM Tris- Hcl, 0.1 mM EDTA, Ph 8). Four µl of this sample was used for PCR (8).
MIRU – VNTR typing
PCR was performed in 25 µl volume that contained 5 to 50 ng of DNA, 0.5 µm of specific primers (Table 1) in the presence of 1.5 m MgCl2, 100 µm of each d NTP, 70 m PCR buffer and 1.25 u recombinant DNA polymerase (Cinagen Co. Iran) (9). DNA was amplified by general PCR. All PCRs were initiated by a 10 min denaturizing step at
72 oC. The temperature cycles for different types of PCRs were as follow: 35 cycles of 1 min at 94 oC, annealing temperatures were used as follow: 61, 60, 64, 67, 65, 64, 61, 64, 58, 63, 62 and 64 for MIRU loci 2, 4, 10, 16, 20, 23, 24, 26, 27, 31, 39 and 40 respectively. Also the MIRU copy numbers in the 12 MIRU-VNTR loci are shown in table 2 (5).
Analysis
Genetic analysis patterns MIRU-VNTR for each strain to a twenty digit profile from right to left indicate loci 2, 4, 10, 16, 20, 23, 24, 26, 27, 31, 39 and 40, respectively. For example pattern of genetic strains H37RV (M. tuberculosis standard strain) a 2, 3, 3, 2, 2, 6, 1, 3, 3, 3, 2 and 1 is the genetic pattern that can be seen in this model in figure 1A (10).
Spoligotyping
Spoligotyping was performed as previously described by Kamerbeek et al (11). The DR region was amplified by PCR using primers derived from the DR sequence. Fifty ?l of the following reaction mixture was used for the PCR: 10 ng of DNA, 20 pmol each of primers DRa and DRb, each deoxynucleoside triphosphate at 200 mM, PCR buffer, and 0.5 u of taq polymerase (Cinagen Co. Iran). The mixture was heated for 2 min at 94 oC and subjected to 30 cycles of 30 sec at 94 oC, 30 sec at 55 oC, and 1 min at 72 oC. The amplified DNA was hybridized to a set of 43 immobilized oligonucleotides, each corresponding to one of the unique spacer DNA sequence within the DR locus.
The sequence of the oligonucleotides used is given in table 2. These oligonucleotides were covalently bound to a hybridization membrane for hybridization. Twenty µl of the amplified PCR product was diluted in 150 µl of 2 Saline – Sodium Phosphate – EDTA (SSPE), supplemented with 0.1% sodium dodecyl sulfate, and heat denatured. The diluted samples (130 µl) were pipetted in to the parallel channels in such a way that the channels of the miniblotter apparatus were perpendicular to the rows of oligonucleotides deposited previously. Hybridization was done for 60 min at 60 oC. After hybridization, the membrane was washed as previously described. Detection of hybridizing DNA was done using a (chemiluminescent ammersh
Result :
As it shown in table 3, 34% of susceptible strains could not identified by MIRU-VNTR, and the remaining 66% were identified as Haarlem family (17%), Dehli/ CAS (17%), NEW1 (14%), and LAM, Uganda and M.X families were 7%. Similarly, 44% drug resistant strains could not be identified by MIRU-VNTR technique. Using both "MIRU-VNTR and Spoligotyping" techniques together significantly reduced the percentage of unknown strains (Table 3).
As shown in table 3, thirteen strains with unknown sample (by "MIRU-VNTR" technique) decreased to 6 strains by using "MIRU-VNTR and Spoligotyping" technique. On the other hand strains (samples specified) increased by using the two techniques; because of the setting of the family samples for some of the unknown strains (Table 3).
Overall by using "MIRU-VNTR" technique, 38% of isolates could not be identified whereas by applying "MIRU-VNTR and Spoligotyping" together only 15% of the isolates remained unknown (p<0.05).
In table 4, the allelic differences for MIRU loci had accounted based on HGDI statistic formula; that is loci 20, 24 and 27 had lower allele difference (HGDI = 0.3), loci 31, 39 and 40 were in middle (0.3 = HGDI = 0.6) and loci 10, 16, 23 and 26 had the most allele difference among the locus MIRU (HGDI = 0.6). Based on table 4 in both sensitive and resistant drug loci 10, 16 and 26 had the most allelic profile between the studied loci. Also loci 2, 4, 20 and 24 had the least allelic difference between all loci.
Discussion :
This study showed that "MIRU-VNTR" had low discriminatory power when it is used alone in comparison to when it is used together with Spoligotyping. Using both techniques together decreased the unknown genetic strains to about 10% in sensitive strains and 20% in drug resistant strains. Therefore, for epidemiological studies we suggest to use "MIRU-VNTR and Spoligotyping" techniques together.
Conclusion :
The results showed that in molecular epidemiologic studies of MTB, using the two techniques of "MIRU-VNTR and Spoligotyping" simultaneously could disseminate the genetic patterns of MTB strains in a much better manner as compared to using only "MIRU-VNTR" technique.
Acknowledgement :
This study was supported by the Mycobacteriology Research Center of Shahid Beheshti University of Medical Sciences.
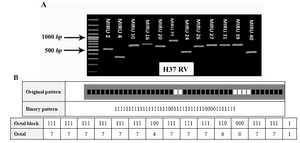
Figure 1. A) PCR products of the various M. tuberculosis H37RV isolates with using primer that amplify 12 locus MIRU-VNTR. Lane M, 100-bp molecular marker. B) Genotypic of M. tuberculosis H37RV. Spoligotyping is from the MIRU-VNTR plus database MIRU-VNTR: Mycobacterial Interspersed Repetitive Units Variable Number Tandem Repeat
|

Figure 2 A) Genotypic and characteristic of resistance and sensitive strains. MIRU-VNTR are from the MIRU-VNTR plus database, MIRU-VNTR; Mycobacterial Interspersed Repetitive Units Variable Number Tandem Repeat; B) Genotypic and other characteristics of resistant and sensitivity strains. Spoligotyping and MIRU-VNTR from the MIRU-VNTR plus database, MIRU-VNTR; Mycobacterial Interspersed Repetitive Units Variable Number Tandem Repeat; RIF: Rifampicin, INH: Isoniazid
|

Table 1. Primer sequence of the MIRU-VNTR loci and Spoligotyping in this study
MIRU-VNTR: Mycobacterial Interspersed Repetitive Unit Variable Number Tandem Repeat
|
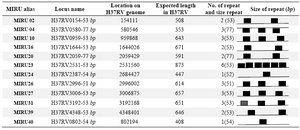
Table 2. MIRU locus information for M. tuberculosis H37RV
MIRU-VNTR: Mycobacterial Interspersed Repetitive Unit Variable Number Tandem Repeat
|

Table 3. Frequency of sensitive and drug resistance strains of MTB by "MIRU-VNTR" assay versus "MIRU-VNTR and Spoligotyping" together
MIRU-VNTR: Mycobacterial Interspersed Repetitive Units Variable Number Tandem Repeat
|
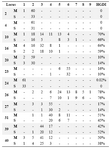
Table 4. Allellic diversity of each MIRU-VNTR locus
MIRU-VNTR: Mycobacterial Interspersed Repetitive Units Variable Number Tandem Repeat; S strain (Susceptible), M strain (Resistant)
|
|