The Potential Anti-inflammatory Effects of Zerumbone in COVID-19 Patients
-
Dehghan, Razieh
-
Research Center for Molecular Medicine, Faculty of Medicine, Hamadan University of Medical Sciences, Hamadan, Iran
-
Azizi Jalilian, Farid
-
Department of Virology, Faculty of Medicine, Hamadan University of Medical Sciences, Hamadan, Iran
-
Najafi, Rezvan
-
Research Center for Molecular Medicine, Faculty of Medicine, Hamadan University of Medical Sciences, Hamadan, Iran
-
 Amini, Razieh
Research Center for Molecular Medicine, Faculty of Medicine, Hamadan University of Medical Sciences, Hamadan, Iran, Tel: +98 813 838 0572; E-mail: aminra14@gmail.com, ra.amini@umsha.ac.ir
Amini, Razieh
Research Center for Molecular Medicine, Faculty of Medicine, Hamadan University of Medical Sciences, Hamadan, Iran, Tel: +98 813 838 0572; E-mail: aminra14@gmail.com, ra.amini@umsha.ac.ir
Introduction :
Dear Editor,
Coronavirus disease 2019 (COVID-19) pandemic started in Wuhan (China) in December 2019 and spread rapidly to become a global health and economic emergency. A powerful inflammatory response to Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) is thought to be the leading cause of disease severity and death in patients with COVID-19, which is associated with high levels of circulating cytokines, profound lymphopenia, and significant mononuclear cell infiltration in different organs like lungs, heart, spleen lymph nodes, and kidney 1. Zerumbone (ZER), which is mainly isolated from the rhizomes of Zingiber zerumbet (Z. zerumbet), was found to be effective against inflammatory and immune disorders. Different studies demonstrated that ZER suppressed the production of pro-inflammatory cytokines in cancerous tissues and several immune cells 2. Therefore, the successful control of inflammatory state would be a promising approach for the management of COVID-19 and the use of therapeutics that modulate inflammation without compromising the adaptive immune response could be the most effective therapeutic strategy. Therefore, the aim of this study was to investigate COVID-19 and ZER relationships through the inflammatory cytokines.
Various studies have reported lung damage and multiple organ failure in subgroups of patients due to robust systemic inflammatory response mediated by cytokine storm 3. Chu et al showed that in vitro human infection with SARS-CoV-2 produces 5 cytokines including IL-6, MCP1, CXCL1, CXCL5, and CXCL10/ IP10 in cell lysate samples from the infected lung tissues 4. Blanco-Melo et al indicated that SARS-CoV-2 decreases IFN-I and IFN-III responses and causes significant induction of several pro-inflammatory chemokines such as IL-1β, IL-6, TNF, and IL-1RA 5. Plasma cytokine and chemokine levels are also elevated in COVID-19 and high levels of plasma IL-6 have been consistently reported in COVID-19 which may increase the risk of poor prognosis and death; therefore, measuring IL-6 levels can be an optimal method to examine patient’s health condition 1.
Some studies revealed that in patients with severe COVID-19 infection, a severe systemic inflammatory reaction has been observed with increased serum levels of inflammatory markers, such as C-Reactive Protein (CRP), Lactic Dehydrogenase (LDH), ferritin, D-dimer, and IL-6, all of which can play a role in cytokine storms initiation 1. Hence, the need for early detection of cytokine storms and the implementation of anti-inflammatory therapy in COVID-19 patients can be an appropriate treatment strategy. Therefore, one strategy for the treatment against COVID-19 is to block the overwhelming inflammation especially in severe cases. Clinical trials are being conducted to assess the inhibition of inflammatory cytokines, including IL-6 and IL-1β 6 and blockade effect of myeloid-like inflammatory cytokine and Granulocyte-Macrophage Colony Stimulating Factor (GM-CSF) has also been assessed 6. ZER is traditionally used to manage many inflammatory disorders including allergies, fever, and asthma 7. ZER significantly suppressed pro-inflammatory cytokines including TNF-α, IL-1β, PGE2, and COX-2 protein in LPS-induced human macrophages through NF-κB, MAPK, and PI3K-Akt signaling pathways. These inflammatory cytokines were also seen in COVID-19 patients with more severe symptoms 7.
Other studies have demonstrated that ZER could attenuate inducible Nitric Oxide Synthase (iNOS) expression which stimulates white blood cells to release IL-1 and TNF and inhibits PGE2 production as well as COX-2 protein expression in macrophages 2.
Different in vivo experiments showed that ZER could significantly suppress IL-1β, IL-6, and TNF-α in plasma in a mouse model of Chronic Constriction Injury (CCI)-induced neuropathic pain 8; moreover, it could reverse the increase in IL-1, IL-6, and TNF-α production in the inflammatory response of diabetic rats 9. ZER could decrease IL-1β, TNF-α, and PGE2 in mice with Acute Ulcerative Colitis (AUC) 10. Also, in male Wistar rats, ZER suppressed IL-6, iNOS activities, and TNF-α concentration 11. In both ovarian and cervical cancer cell lines, ZER significantly reduced IL-6 levels 12.
Hypoxia is present in severe pneumonia and respiratory distress 13. HIF-1α is a critical factor in hypoxic conditions which by activating STAT3 could lead to an increase of IFNI, IL-6, and TNF-α in the inflammatory process. Therefore, suppression of HIF-1 transcription or inhibition of its activity can be effective in reducing inflammation caused by COVID-19 viral infection in affected organs 13. It was found that in hypoxic conditions, ZER could reduce metastasis in cancer cells through reducing CXCR4 and HIF-1A 14. ZER exerts anti-bacterial and anti-inflammatory effects by decreasing the expression of IL-17A, TNF-α, Keratinocytes-derived Chemokine (KC), and iNOS in colonic tissues of Enterotoxigenic Bacteroides fragilis (ETBF)-infected mice through the inhibition of NF-κB signaling pathway 15. Also, ZER could be used as a drug due to its immunomodulating activity in modulating the cytokine expression such as human interleukin-12p70 in chronic infections and various autoimmune disorders 16.
Altogether, it can be suggested that ZER could attenuate the cytokine storm in COVID-19 through upregulation of IL-6, IL-1β, and TNF-α expression which was abolished in COVID-19 patients (Figure 1).
Conflict of Interest :
There is no conflict of interest.
Funding: This study was supported by a grant from Hamadan University of Medical Sciences, Hamadan, Iran (No. 990223950).
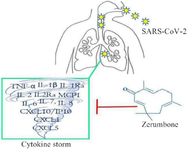
Figure 1. ZER could attenuate the cytokine storm in COVID-19 patients through the suppression of pro-inflammatory cytokines.
|
|