Molecular Mechanisms of Anti-inflammatory Activities of the Extracts of Ocimum gratissimum and Thymus vulgaris
-
Francis Olaoye, Ige
-
Department of Biochemistry, McPherson University, Seriki Sotayo, Ogun State, Nigeria
-
 Joseph Oso, Babatunde
Department of Biochemistry, McPherson University, Seriki Sotayo, Ogun State, Nigeria, Tel: +23 48060625697; E-mail: basjoe08@gmail.com
Joseph Oso, Babatunde
Department of Biochemistry, McPherson University, Seriki Sotayo, Ogun State, Nigeria, Tel: +23 48060625697; E-mail: basjoe08@gmail.com
-
Aberuagba, Adepeju
-
Department of Biochemistry, University of Ilorin, Ilorin, Kwara State, Nigeria
Abstract: Background: A large body of literature suggests that the extracts of Ocimum gratissimum (O. gratissimum) and Thymus vulgaris (T. vulgaris) play protective roles against various inflammatory disorders. However, the possible mechanism of action with reference to the interactions of their respective phytochemical compositions with pro-inflammatory mediators as the indication of their therapeutic effects is less clear. Therefore, the immunomodulatory properties of O. gratissimum and T. vulgaris were investigated in this study.
Methods: The in vitro lipoxygenase inhibitory potentials of methanolic extracts of the selected plants were assessed through colorimetric analysis. The pharmacokinetics of some identified compounds in the botanicals were investigated via the Swiss ADME server while the molecular interactions of the compounds with lipoxygenase, IL-1, IL-6, TNF-α, IL-8, and CCL-2 were performed through molecular docking.
Results: The assessment of the lipoxygenase inhibition revealed the extracts could possess anti-inflammatory agents. The pharmacokinetic results of some selected compounds identified in the botanicals showed moderate toxic effects compared to indomethacin. The molecular docking study substantiated the report of the in vitro analysis as indicated in the binding score of all the selected compounds compared to indomethacin.
Conclusion: The phytochemical components of the extracts of O. gratissimum and T. vulgaris could be effective as anti-inflammatory agents that could be explored in preventing disorders associated with excessive activities of pro-inflammatory mediators.
Introduction :
One of the most promising recent alternatives to classical treatment is the use of immunomodulators for the prevention of diseases that could be associated with dysregulated inflammatory responses. Numerous clinical documents evidenced that many pathological conditions closely related to impaired inflammatory responses mediated by cytokines and chemokines play a role in the pathogenesis of many infections such as nephritis, rheumatoid arthritis, uveitis, early stages of insulin-dependent diabetes mellitus, and thyroiditis 1,2. Similarly, high expression levels of IL-1β, IFN-γ, IP-10, and monocyte chemoattractant protein 1 (or CCL-2) have been observed in COVID-19 patients with the severity of the disease corresponding to the serum levels of IL-2R and IL-6 in some patients 3. Other reports revealed that COVID-19 patients in the Intensive Care Unit (ICU) had high serum levels of granulocyte colony-stimulating factor, IP-10, MCP-1, macrophage inflammatory protein-1A, and TNF-α, suggesting that cytokine storm positively correlates with the disease severity. One of the major concerns that could be associated with the down-regulation of inflammatory activities is the suppression of the immune response to the pathogenic organisms; however, regulation of pro-inflammatory activities through inhibition of the biological effects of pro-inflammatory cytokines could reduce pathological deterioration associated with inflammatory disorders. Additionally, targeting the enzymes of the eicosanoid pathway such as Cyclooxygenase 2 (COX-2) and Lipoxygenase (LOX) could therapeutically provide benefit in the context of pathogenesis of certain inflammatory diseases as these enzymes play important roles in pathophysiology following pathogenic infection and release of potent pro-inflammatory cytokines and chemokines 4. Dietary modulation of the inflammatory responses and immune capacity are important tools to cope with twin maladies of excessive inflammatory reactions and suppressed immune system.
Research has focused on the exploration of efficient phytochemicals that can be used for the prevention of diseases and/or treatment purposes. Certain plant parts have been reported to inhibit the activities of pro-inflammatory mediators 5,6. Examples of such botanicals include the leaves of Ocimum gratissimum (O. gratissimum) and Thymus vulgaris (T. vulgaris), well-known herbs in the tropical and subtropical regions widely used as medicinal plants and food additives due to their health benefit potentials 7. Despite frequent and regular use of these herbs, the possible mechanism of actions with reference to the interactions of their respective phytochemical compositions with pro-inflammatory mediators, indicating their therapeutic effects, is less clear. Thus, this study investigated the putative molecular mechanism of anti-inflam-matory effects of O. gratissimum and T. vulgaris.
Materials and Methods :
Chemicals: All the chemicals used were of analytical grade. Methanol, sodium dihydrogen phosphate, and disodium hydrogen phosphate were products of Guangzhou JHD Chemical Reagent Co., Ltd. Guangzhou, China.
Extracts preparation: The leaves of O. gratissimum and T. vulgaris were obtained from Ogunmakin market, Obafemi/Owode, Ogun State, Nigeria and authenticated at the Department of Biochemistry, McPherson University, Seriki Sotayo, Ogun State, Nigeria. The samples were air-dried at room temperature of 30±1°C. Exactly 50 g of each dried samples were pulverized and soaked in 100 ml of absolute methanol for 48 hr at room temperature of 30±1°C. The samples were filtered in Whatman TM No 1 and the filtrate was allowed to stand at room temperature of 30±1°C for the removal of the solvent. The obtained extracts were used for the analysis of lipoxygenase inhibitory potential.
Lipoxygenase inhibitory potential: The lipoxygenase inhibitory potentials of varying concentrations (between 20 µg/ml and 100 µg/ml) of methanolic extracts of O. gratissimum and T. vulgaris were determined as described by Shinde et al and Sorkun et al 8,9, using linoleic acid as the substrate and crude lipoxygenase prepared from soybean as previously reported by Oso and Karigidi 10 as the source of the enzyme. Accurately, 0.1 ml of each extract was added to a test tube containing 0.5 ml of 0.1 M phosphate buffer (pH=9.0) and 150 µl of the enzyme, lipoxygenase. The mixture was allowed to incubate at 29°C for 5 min. Afterwards, 0.5 ml of 0.6 mM linoleic acid solution was added to the mixture. The absorbance was measured at 234 nm. The results were presented as the values of percentage inhibition of lipoxygenase activity.
Phytochemical identification and ADMET properties of phytochemical contents: The major phytochemical contents of O. gratissimum and T. vulgaris were retrieved from Dr Duke's Phytochemical and Ethnobotanical Databases. Theoretical physicochemical properties of the identified compounds, as well as their corresponding ADME parameters, pharmacokinetic properties, drug-like nature, and medicinal chemistry friendliness of the selected compounds were predicted using the Swiss ADME server (http://www.swissadme.ch/index.php) 11.
Molecular docking: The 3D X-ray crystal structure of lipoxygenase was retrieved from the Protein Data Bank (https://www. rcsb.org/) with PDB ID: 3D3L. The crystal structure was prepared by removing the existing ligand using the DS BIOVIA tool. Similarly, the 3D crystal structures of the selected cytokines of IL-1, IL-6, TNF-α, IL-8, and CCL-2 with PDB IDs: 3NJ5, 4NI7, 1TNR, 6N2U, and 4ZK9, respectively were obtained and prepared for molecular docking. The 3D structure of identified major compounds in O. gratissimum and T. vulgaris were obtained from Zinc15 (http://zinc15.docking.org) in SDF format 12 and converted to PDB format using BIOVIA DS Visualizer. Each protein with all ligands on PyRx virtual screening was docked and the respective binding scores were obtained for each ligand 13. The docked files for each protein-ligand interaction were viewed using the DS BIOVIA software tool to obtain the 2D and 3D views of the complexes. All the docked cytokines were combined with all ligands separately in PDB format using PyMOL ver. 1. 1eval (DeLano Scientific LLC, USA) for molecular dynamics study.
Molecular dynamics simulation: The conformational stability of the lipoxygenase-ligand complexes and the pro-inflammatory cytokines-ligand complexes obtained from the molecular docking was assessed. This assessment was done using molecular dynamics simulations analysis performed through iMODS server (http://imods.chaconlab.org) by normal Mode Analysis (NMA) in internal coordinates (Torsional space) predicting properties such as deformability, mobility profiles, eigenvalues, variance, co-variance map and elastic network of the protein-ligand interactions 14.
Statistical analysis: The results were analyzed using a One-way Analysis of Variance (ANOVA) for mean differences among the various extracts followed by Duncan’s multiple range tests for post hoc comparisons at p<0.05 and presented as means±standard deviation of three determinations.
Results and Discussion :
In vitro lipoxygenase inhibition study: Inflammation is known as a normal biological process as a result of tissue injury, viral/microbial pathogen infection, and chemical irritation. Over-expression of LOX and the pro-inflammatory metabolites of arachidonic acid such as leukotrienes have been linked to many human pathological conditions such as inflammation, cardiovascular diseases, and cancer 15,16. The result of lipoxygenase percentage inhibition potential of the plant extracts compared to indomethacin reveals that O. gratissimum showed the least potential while indomethacin exhibited the highest potential at all doses (Figure 1). Indomethacin, a non-steroidal anti-inflammatory drug, is widely used in the management of inflammatory diseases 17. However, the in vitro study revealed that indomethacin has higher percentage inhibition towards LOX compared to the two plant extracts. The extracts of O. gratissimum and T. vulgaris showed inhibition properties at all doses suggesting their anti-inflammatory potential which could inhibit the rate-limiting step in arachidonic acid metabolism thus reducing the synthesis of leukotrienes. This finding corresponds to the previous reports of Wei and Shibamoto 18 on LOX inhibitory effects by T. vulgaris and O. gratissimum.
Assessment of molecular mechanism of the LOX inhibitory potentials of O. gratissimum, T. vulgaris, and indomethacin through structure-based virtual screening revealed that indomethacin had the highest binding affinity due to its least binding score which is significantly different from all the phytocompounds identified in O. gratissimum and T. vulgaris through Dr Duke's Phytochemical and Ethnobotanical Databases (Table 1). However, all the investigated compounds had good interactions with LOX based on their negative values 19.
These binding interactions are mainly due to pi-interactions between compounds and LOX as well as hydrogen bonds in all the evaluated compounds (Figure 2). Although the number of hydrogen bond interactions is low compared to the binding score, the high binding score could be as a result of pi-interactions from Val190, leu178, leu227, leu361, and Phe352, His360, and His365 via promotion of better position and organization of the proteins 20,21. The selected compounds in T. vulgaris (Cymene, terpinene, and thymol) showed no significant difference in the binding score and interacted with His360 and His365. Conversely, indomethacin did not interact with His360 and His365; however, its high binding score with LOX could be due to interactions with several residues through covalent interactions such as a carbon-hydrogen bond, pi-pi stack, pi-alkyl, van der Waals with polar amino acids such as Gln435, His462, Asp512, and Arg641 and non-polar amino acids of Leu178, Ala433, Ala434, Leu447, Leu453, Pro456, Ala461, Leu465, Leu507, and Cys508. The interactions such as pi-pi stack, pi-alkyl, van der Waals between the hydrophobic residues and LOX could be responsible for the stability and activity of the proteins 22.
Pharmacokinetic study: The in silico pharmacological assessments of the drug-likeness of the identified phytocompounds were carried out through SwissADME server. The solubility features revealed that all the compounds had consensus Log p-values less than 5 like their lipophilicity feature. Interestingly, all the compounds were soluble in water by Log S (ESOL), Log S (Ali), and Log S (SILICOS-IT) analysis except terpinene which was moderately soluble by Log S (Ali) analysis (Table 2). The results revealed equal lipophilicity suggesting a better absorption and permeability of the selected compounds in living organisms due to less than 5 consensus Log P values 23. In line with this, the prediction of water solubility using Sorkun and Khetan’s 9 report on three different models showed that none of the evaluated compounds’ Log S values exceeded -4.00 except terpinene with Log S (Ali) value of -4.220 (0.008 mg/ml) suggesting better absorption and metabolism as a result of excellent water solubility. Also, caryophyllene oxide, ocimene, and eugenol showed high gastrointestinal absorption as revealed by their low affinity for permeability-glycoprotein except for ocimene. Meanwhile, cymene, terpinene, and thymol showed low gastrointestinal absorption except for thymol with a low affinity for permeability glycoprotein.
Additionally, the results of the pharmacokinetics and the interactions of the selected phytocompounds with P-glycoprotein (P-gp) and cytochromes P450 (CYPs) and the predicted drug-likeness are presented in table 3. Caryophyllene oxide and eugenol showed high GI absorption except for ocimene while cymene and terpinene showed low GI absorption except for thymol. All selected compounds irrespective of the plant's source can cross the blood-brain barrier and showed skin permeation values greater than -2.5 cm/s while none served as the P-gp substrate. In a similar pattern, all the compounds showed no inhibitory effect on CYP2C9 and CYP2C19 suggesting that all compounds except caryophyllene oxide could not hinder the synthesis of epoxyeicosatrienoic acids (an anti-inflammatory agent) and terminate the metabolism of therapeutic drugs like anti-ulcer, anti-malaria, anti-convulsant, anesthetic, and sedative drugs 24. In an almost similar pattern, only cymene could inhibit CYP2D6 and stop the metabolism of anti-hypersensitive and anti-arrhythmic drugs as well as β-blockers and anti-depressants. The drug-likeness appraised based on five different rule-based filters showed that none of the selected compounds violated the Lipinski, Egan, and Veber rules. However, all the compounds violated the Ghose and Muegge rules with lower molecular weight except for caryophyllene oxide and eugenol with molecular weights greater than 160 g/mol 11.
The radar plots of all the compounds were within the physicochemical ranges which indicate the suitability of the compounds through oral administration (Figure 3). This computation largely correlates with their observed lipophilicity and water-solubility properties.
Molecular docking study of cytokines: A relationship has been established between the pathogenic expressions of LOX and cytokine production as LOX inhibitory agents had been shown to ameliorate Tumor Necrosis Factor-α (TNF-α)-induced cytokine and chemokine release 25,26. This indicates that LOX inhibitors may be better candidates for the treatment of inflammatory diseases that are associated with cytokine storms. The molecular docking analyses between the selected phytocompounds and some pro-inflammatory mediators such as interleukin 1, interleukin 6, TNF-α, interleukin 8, and monocyte chemoattractant protein-1 (or C-C chemokine ligand 2, CCL-2) revealed that the phytochemicals had a reasonable binding affinity for the pro-inflammatory mediators (Table 4) suggesting the modulatory potential of the compounds. These inflammatory mediators play important roles in the natural responses to pathogenic threats and the development of pathological disorders which could lead to chronic inflammation characterized by an exaggerated cytokine "storm" and the release of damage-associated molecular patterns 27. It could be concluded from figure 4 and table 4 that the significant low binding scores observed in cis-ocimene and terpinene interaction with IL-1 might be due to absence of Leu113 in the interacting site.
The residue Pro129 was shown to be a probable and important residue required for IL-1 activity as it was found to interact with all the selected phytocompounds (Figure 4). Caryophyllene oxide had the highest binding affinity with IL-1, IL-6, and TNF-α. This could be due to the contribution of Van der Waals forces to the interaction between the cytokines. IL-1, IL-6, and TNF-α are potent cytokines involved in various human diseases 28. Moreover, TNF-α could also stimulate the expression of IL-1 and IL-6, causing the associated pathological disorders 29. Various studies had provided a rationale to explore their respective targets in the development of therapy for certain diseases associated with autoimmune disorders 30. This could prevent the respective cascade of signaling processes through the JAK/STAT3 activation pathway and transcription of genes of inflammatory proteins including cytokines, receptors, and protein kinases 31. Eugenol had the best binding affinity followed by thymol with IL-8. His16 was identified as an important residue in the interaction of the chemokine with the phytocompounds. IL-8, a chemoattractant usually released by macrophages, induces inflammation through the recruitment of neutrophils and other immune cells to the site of injury or infection 32. IL-1 and TNF-α could also induce IL-8 production from a variety of cells 33. Cymene and eugenol depicted the highest binding affinity towards monocyte C-C chemokine Ligand 2 (CCL-2) followed by thymol. The observed binding scores might be due to the pi-pi stacking interactions of the compounds with CCL-2 through Ile20, Ala53, and Val60 (Figure 4). Similarly, the affinity of thymol with CCL-2 could be associated with the conventional hydrogen bond formed between thymol and CCL-2 34. Excessive inflammation connected with various diseases such as autoimmune disease, atherosclerosis, and neurological disorders had been linked with the production of CCL-2 and its interaction with its main chemokine receptor CCR2 35. CCL2 has been assumed to be a therapeutic target as its inhibition was found to decrease lung inflammation, metastases, and atherosclerosis in animals 36.
Molecular dynamics study: The effects of the identified phytocompounds on the flexibility of the cytokines and the chemokines upon binding were assessed with molecular dynamics simulations derived factors such as deformability, B-factor, eigenvalues, variance, covariance, and elastic factors considered in the study (Figure 5). The interactions of the phytocompounds with the cytokines and chemokines did not induce changes in the flexibility and dynamics of the pro-inflammatory mediators. However, there were variances in the flexibility and dynamics of the pro-inflammatory cytokines and chemokines. The main-chain deformability was higher in the CCL-2 while IL-8 had the highest rigidity among the selected mediators. The energy required to deform the TNF-α is relatively high as indicated by the lowest eigenvalue (2.741269e-05) (Figure 5C). However, IL-1 had the lowest variance associated with each normal mode which is inversely related to the eigenvalue (Figure 5D). The interaction between pairs of residues of the pro-inflammatory mediators computed using the Cα Cartesian coordinates showed that the cytokines (IL-1, IL-6, and TNF-α) could experience moderately correlated motion between the residues comparable to the chemokines (IL-8 and CCL-2) (Figure 5E). The stiffness was found to be comparatively lower in IL-8 and CCL-2 as indicated by the lesser distribution of dark grey dots in the map (Figure 5F) 14,37.
Conclusion :
This study showed that all the selected phytochemical components of O. gratissimum and T. vulgaris except cis-ocimene interacted adequately with the selected inflammatory mediators. Thus, repositioning these compounds as pro-inflammatory antagonists could be a promising strategy to alleviate inflammatory disorders that could arise from infectious diseases or dysfunctional immune disorders. Further analyses are recommended to confirm the putative therapeutic effects of the selected compound.
Acknowledgement :
We recognize and appreciate the assistance of the staff of the Department of Biological Sciences, McPherson University, Seriki Sotayo.
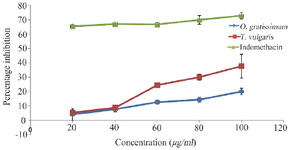
Figure 1. Lipoxygenase inhibitory potentials of O. gratissimum and T. vulgaris comparable to indomethacin.
|
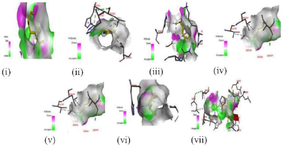
Figure 2. 3D illustration of the molecular interactions of (i) caryophyllene oxide, (ii) cymene, (iii) eugenol, (iv) cis-ocimene, (v) terpinene, (vi) thymol, and (vi) indomethacin with lipoxygenase.
|
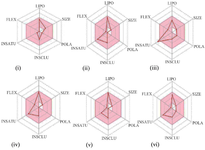
Figure 3. Suitable physicochemical space for oral bioavailability of (i) caryophyllene oxide, (ii) cymene, (iii) eugenol, (iv) cis-ocimene, (v) terpinene, and (vi) thymol.
|

Figure 4. 3D illustration of the molecular interactions of (i) caryophyllene oxide, (ii) cymene, (iii) eugenol, (iv) cis-ocimene, (v) terpinene, and (vi) thymol with, A) IL-1, B) IL-6, C) TNF-α, D) IL-8, and E) CCL-2.
|

Figure 5. Molecular dynamics simulation of (i) IL-1, (ii) IL-6, (iii) TNF-α, (iv) IL-8, and (v) CCL-2 showing the, A) Deformability, B) B-factor, C) Eigenvalues, D) Variance, E) Covariance map, and F) Elastic network.
|
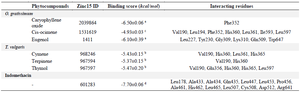
Table 1. Molecular interactions of some selected phytochemical components of T. vulgaris and O. gratissimum with lipoxygenase
a-d: Values with different superscripts are significantly different at p<0.05.
|
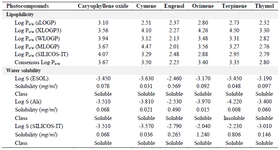
Table 2. Solubility characteristics of the selected phytocompounds
|
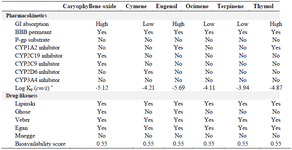
Table 3. The pharmacokinetics properties of the selected compounds
* Skin permeation.
|
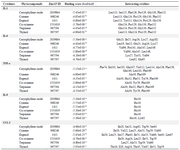
Table 4. Molecular interactions of some selected phytochemical components of T. vulgaris and O. gratissimum with pro-inflammatory cytokines
a-f: Values with different superscripts are significantly different at p<0.05.
|
|