Apoptosis Induction with Combined Use of Cisplatin and Fisetin in Cisplatin-resistance A2780 Ovarian Cancer Cells
-
Jafarzadeh, Samira
-
Department of Biology, Mashhad branch, Islamic Azad University, Mashhad, Iran
-
 Baharara, Javad
Department of Biology, Mashhad branch, Islamic Azad University, Mashhad, Iran, E-mail: baharara@mshdiu.ac.ir
Baharara, Javad
Department of Biology, Mashhad branch, Islamic Azad University, Mashhad, Iran, E-mail: baharara@mshdiu.ac.ir
-
Research Center for Animal Development Applied of Biology, Mashhad branch, Islamic Azad University, Mashhad, Iran
-
Tehranipour, Maryam
-
Department of Biology, Mashhad branch, Islamic Azad University, Mashhad, Iran
Abstract: Background: Ovarian cancer is the leading cause of death caused by genital cancers. One of the most common treatments for this type of cancer is chemotherapy by cisplatin, which induces apoptosis in cancer cells. Apoptosis is a type of physiological cell death. Cisplatin chemotherapy usually has several side effects and cellular resistance to cisplatin is a common incidence. In order to overcome these problems, the use of combination therapies using natural substances has been considered. Fisetin is a flavonoid with anti-cancer activity which induces apoptosis. In this study, the apoptosis induced by cisplatin along with Fisetin in cisplatin-resistant ovarian cancer cell line (A2780) was investigated.
Methods: In the present experimental study, the effect of combined use of Fisetin and cisplatin on ovarian cancer cell lines (A2780) was investigated by using MTT assay. Cell death was also determined by DAPI, acridine orange/propidium iodide, and Annexin/PI assay. Apoptotic gene expression of Bax, BCL-2, caspase 3, and caspase 9 was also assessed by real time PCR.
Results: The results of MTT assay indicated that the combined treatment of Fisetin and cisplatin effectively inhibits proliferation of A2780 cells. The results of DAPI staining showed that fragmentation of chromatin in cells occurred in the combined treatment. Acridine orange-propidium iodide staining and Annexin/PI staining showed an increase in the rate of apoptotic cells in cells under combined treatment. The results of the study regarding changes in gene expression also indicated that Bax pro-apoptotic gene expression and BCL-2 anti-apoptotic gene expression increased in cells under treatment; moreover, gene expression of caspases 3 and 9 significantly increased as well.
Conclusion: According to the findings of this study, the combined use of cisplatin and Fisetin increases the induction of apoptosis in cisplatin-resistant ovarian cancer cells (A2780); therefore, the combined use of cisplatin and Fisetin can be considered a promising strategy in the treatment of ovarian cancer.
Introduction :
Cancer is the second leading cause of death in the world after cardiovascular diseases. Half of men and one-third of women in the United States develop cancer in their lifetime. Current cancer treatments include methods such as surgery, chemotherapy, and radiation therapy, of which chemotherapy is still the primary method of cancer control 1. Most chemotherapy drugs including Doxorubicin, Vincristine, Cyclophosphamide, Topotecan, and Paclitaxel select cancer cells based on their high proliferative capacity, which can lead to increased toxicity in normal tissues such as bone marrow, gastrointestinal tract, and hair follicles which are highly proliferative; therefore, low doses are often prescribed in anti-cancer treatment strategies, resulting in ultimate failure to treat the tumor 2. Other harmful effects of chemotherapy include the development of resistance in cancer cells, and ultimately tumor regrowth and increased mortality 3.
Drug resistance in cancer cells is a complicated challenge and most likely depends on cell tissue and microenvironment 4. For example, cisplatin (Chemotherapy drug) is one of the first-line treatments for physicians against tumors, especially lung, ovarian, and testicular cancers. Although cisplatin is usually effective in the first injection, its application in treatment process has a major drawback, namely, low efficiency and resistance to cancer cells 5. Therefore, in order to overcome drug resistance, new therapies with greater effectiveness and fewer side effects should be considered. In this regard, the use of polytherapy to overcome drug resistance in cancer cells is the top strategy 6.
Herbal compounds as natural cancer inhibitors are an emerging and attractive strategy for cancer management, while fruits and vegetables are a rich source of cofactors, vitamins, and minerals. Phytochemicals, including flavonoids, have a special ability to target important cellular events involved in cancer development 7. Many studies have shown that herbal compounds have antioxidant, anti-inflammatory, and anti-cancer properties 8. 3′, 4′, 7-Tetrahydroxy is a natural flavonoid, commonly known as Fisetin, which is found in fruits and vegetables such as strawberries, apple, persimmon, grapes, onion, and cucumber 9.
Fisetin has been reported to inhibit cell cycle in HT-29 human colorectal cancer cells 10. It has also been shown to have an antiproliferative effect on prostate cancer cell lines 11. Fisetin decreases the proliferation and increases apoptosis in tumor cells such as prostate cancer, liver cancer, colon cancer, pancreatic cancer, hepatocellular carcinoma, and leukemia cells by increasing or decreasing genes involved in apoptosis 12. In this study, in order to use polytherapy, the effect of combined use of cisplatin and Fisetin to overcome cell resistance in cisplatin-resistant A2780 cell line and the occurrence of cell death at low concentrations of cisplatin were evaluated.
Materials and Methods :
Cisplatin-resistant A2780 cells were obtained from Pasteur Institute of Iran. RPMI-1640 growth medium, Fetal Bovine Serum (FBS), penicillin-streptomycin, trypsin, DAPI dye, acridine orange, propodium iodide, and MTT powder were prepared (Sigma-Aldrich, UK). Cisplatin was purchased from Mylan Company in France and Fisetin was purchased from Sigma Aldrich in Germany. RNA extraction kit, cDNA synthesis kit, and SYBR Green were purchased from Pars tous Company in Iran and proprietary primers were purchased from Bioneer Corporation in South Korea.
Cell culture and maintenance: Cells of A2780 cell line were cultured in RPMI-1640 growth medium containing 10% FBS and 1% penicillin-streptomycin in cell culture flasks and were kept in incubator at 37°C with %5 CO2 and 95% humidity. Cells were used for tests when reaching a density of 90% 13.
MTT assay: For MTT assay, 5×104 cells were cultured in 96-well plates. After 24 hr, A2780 cells were treated with cisplatin concentrations of 0.1, 0.5, 0.75, and 1 μg/ml and Fisetin concentrations of 50, 75, 100, 125, 150, 200 μg/ml. In order to evaluate the effects of combined use of cisplatin and Fisetin, A2780 cells were treated with Fisetin concentrations of 75 and 100 μg/ml and cisplatin concentrations of 0.1 and 0.5 μg/ml; no treatment was performed on A2780 ovarian cancer cells as the control group. After 24 hr of treatment, 10 μl of 5% MTT solution was added to the cells and the cells were incubated for 3 to 4 hr in the dark at 37°C. An amount of 80 μl of DMSO was added and the plate was read by spectrophotometer (BioRad, US) at 560 nm. Rate of viability of cells under treatment was calculated using the following formula:
Viability rate=(100* control absorption/sample absorption)
For each concentration, the cycle was repeated up to 6 times.
DAPI nuclear staining: To carry out this test, 5×104 ovarian cancer cells of A2780 cell line were cultured in 6-well plates; after 24 hr, based on IC50 values, the combined concentration including 0.1 μg/ml cisplatin and 50 μg/ml Fisetin was used for treatment. After 24 hr, the cells were fixed with methanol and stained with DAPI using fluorescence microscope (Olympus, Japan), and the cells’ morphology was examined 14.
Acridine orange/propidium iodide staining: To perform this test, 5×104 ovarian cancer cells of A2780 cell line were cultured in 6-well plates; after 24 hr, based on IC50 values, a combined concentration including 0.1 μg/ml cisplatin and 50 μg/ml Fisetin was used for treatment. After 24 hr, the cells were detached from the bottom of the plate with trypsin and centrifuged and then 10 μl of PI and 10 μl of acridine orange were added to the cells. After placing the coverslip on the slide, the cells were examined using a fluorescence microscope (Olympus, Japan) 15.
Annexin V-FITC/PI assay in different treatment groups: To perform this assay, 5×104 ovarian cancer cells of A2780 cell line were cultured in 6-well plates. After 24 hr, based on IC50 values, a combined concentration including 0.1 μg/ml cisplatin and 50 μg/ml Fisetin was used for treatment. Over the following 24 hr, the cells were separated from the bottom of the plate and centrifuged. Then, according to the kit manufacturer's protocol, 5 μl of Annexin V-FITC and 5 μl of propidium iodide were added to each sample. The samples were incubated at ambient temperature in darkness for 5 min and evaluated by flow cytometry (Debe, USA). The data were analyzed using the device software 16.
Genes expression changes in BCL-2, Bax, Casp3, and Casp9 using real time PCR: The expression level of BCL-2, Bax, Casp3, and Casp9 genes was measured using real time PCR. To this purpose, 106 A2780 cells were cultured in a 2.5 ml flask. After 24 hr, based on IC50 value, a concentration including 0.1 μg/ml cisplatin and 50 μg/ml Fisetin was used for cell treatment. After 24 hr, using RNA extraction kit and based on Triazole method, RNA of cells was extracted. Then, using the Easy cDNA Synthesis Kit (Pars tous, Iran) and according to its instructions, cDNA synthesis was performed. In order to perform real time PCR for BCL-2, Bax, Casp3, and Casp9 genes, specific primers and master mixes containing SYBR Green were used. The cycle was repeated up to 30 times and multiple copies of the gene were produced. Its amount was determined and recorded by the detector and the results were analyzed.
Statistical analyses: In this study, quantitative data were analyzed using SPSS software v22 (IBM, USA) and t-test was performed at a significance level of p<0.05.
Results :
Evaluation of survival rate of A2780 cells treated with cisplatin, Fisetin, and combined use of cisplatin and Fisetin with MTT assay: Comparing experimental groups with the control group, it was revealed that the rate of viability of A2780 ovarian cancer cells treated with cisplatin decreased with concentration. Cell viability decreased by 50% when the concentration level was 0.75 μg/ml. Concentrations lower than IC50 were used to evaluate synergistic effects. The effect of Fisetin on toxicity of A2780 ovarian cancer cells also indicated that the substance decreased the viability of cells in 24 hr in a concentration-dependent manner, so that it had no significant effect on cell viability at concentrations lower than 100 μg/ml; the concentration which caused the death of 50% of cells was approximately equal to 150 μg/ml. The use of higher concentrations led to the death of A2780 ovarian cancer cells. In the experimental groups, which were treated simultaneously with Fisetin and cisplatin, in all concentrations, cell death significantly increased with the application of each of these substances alone and the rate of cell viability in all groups was less than 50% (Figure 1).
Evaluation of apoptosis induction by DAPI nuclear staining: The result of staining shows that the use of cisplatin and Fisetin alone can induce nuclear fragmentation of A2780 cells. However, considering the use of Fisetin and cisplatin at certain concentrations with low toxicity, the effect of apoptosis is not significant. The results of this study showed that the combined use of cisplatin and Fisetin can increase the effects of apoptosis in cells under treatment, so that the number of apoptotic cells observed with bright color in the nucleus increased compared to the application of cisplatin and Fisetin alone (Figure 2).
Evaluation of apoptosis induction of acridine orange/ propidium iodide staining: Acridine Orange (AO) and Propidium Iodide (PI) are nuclear staining agents (Nucleic acid). AO is permeable to both living and non-living cell types and produces a bright green color in living cells. PI produces red fluorescence and enters only dead cells in which cell membrane is entierly destroyed. This type of staining was used to examine live and dead cells in different treatment groups. The results showed that in A2780 cells treated with only cisplatin and only Fisetin, the number of apoptotic cells was relatively small compared to the control group, so that most cells were green and healthy. However, in combined groups, the number of red cells with cell death was higher than the number of cells treated with either Fisetin and cisplatin alone (Figure 2).
Evaluation of apoptosis induction by Annexin V-FITC/PI assay in different treatment groups: The results of this assay showed that more than 85% of the cells were alive in the control group. In the experimental group, with a concentration of 0.1 g/ml of cisplatin, about 37% of the cells were alive and about 6% of the cells had primary apoptosis. More than 42% of the cells had secondary apoptosis and about 14% of the cells had necrosis. In the experimental group, where the cells were treated with a concentration of 50 g/ml of Fisetin, about 36% of the cells were alive and more than 57% of the cells had secondary apoptosis. In the combined group where a concentration of 0.1 μg/ml of cisplatin and 50 μg/ml of Fisetin was used, more than 80% of the cells had secondary apoptosis (Figure 2).
Changes in BCL-2, Bax, caspase 3, and caspeas 9 gene expressions in different treatment groups: While BCL-2 gene expression in A2780 treated cells with 0.1 μg/ml cisplatin concentration increased 17.5 times compared to the control group, in the group with 50 μg/ml Fisetin concentration, an increase of 1.5 times compared to the control was observed. In cells with a combined concentration of 0.1 μg/ml cisplatin+ 50 μg/ml Fisetin, an increase of 2.5 times compared to control was observed (Figure 3).
Regarding Bax gene expression for the cells treated with 0.1 μg/ml cisplatin concentration, an increase of 25 times in gene expression compared to control was observed. In cells treated with 50 μg/ml Fisetin concentration, an increase of 2 times in gene expression was observed compared to the control. In the cells treated with a combined concentration of 0.1 μg/ml cisplatin+ 50 μg/ml Fisetin, an increase of 5.81 times compared to the control was observed. Regarding caspase 3 gene expression for the cells treated with of 0.1 μg/ml cisplatin concentration, an increase of 15 times in gene expression compared to the control was observed. In the cells treated with 50 μg/ml Fisetin concentration, an increase of 1.3 times in gene expression compared to the control was observed.
In the cells treated with combined concentration of 0.1 μg/ml cisplatin+50 μg/ml Fisetin, an increase of 2.6 times in gene expression compared to the control was observed. Regarding caspase 9 gene expression, in cells treated with 0.1 μg/ml cisplatin concentration, an increase of 15 times in gene expression compared to the control was observed. In the cells treated with 50 μg/ml Fisetin concentration, an increase of 1.35 times in gene expression compared to the control was observed. In the cells treated with a concentration of 0.1 μg/ml cisplatin+50 μg/ml Fisetin, an increase of 5.5 times in gene expression compared to the control group was observed.
Discussion :
In the present study, different methods were used to investigate the effect of combined use of cisplatin and Fisetin on induction of apoptosis in cisplatin-resistant cancer cells in the ovary (A2780 cell line). MTT assay was used to assess the toxicity. The results showed that cisplatin and Fisetin in a concentration dependent manner caused the death of A2780 cancer cells. The results showed that the combined use of these two substances significantly decreases the viability of cells at concentrations lower than IC50 of cisplatin and Fisetin; therefore, in all combined concentrations, the rate of cell viability was lower than 30%. Considering this result, the minimum effective concentration combining 50 μg/ml of Fisetin and 0.1 μg/ml of cisplatin to further decrease the toxicity of cisplatin is recommended.
In a similar study, Chen et al showed that although treatment of cisplatin-resistant ovarian cancer cells (A2780) with cisplatin alone did not decrease the growth and proliferation of these cells, the combined use of cisplatin and the natural substance of berberine hindered growth and the induction of cell death in the groups under treatment 17. In a study conducted on the effect of cayenne pepper in combination with cisplatin and also paclitaxel on increasing apoptosis in ovarian cancer cells, Gong et al showed that in combined treatment of cayenne pepper with any of the cisplatin or paclitaxel chemotherapeutic drugs, the rate of cancer cell viability relative to the use of each drug alone decreases in a dose-dependent manner which was consistent with the results of our study on the reduction of ovarian cancer cell viability in cisplatin and Fisetin combined treatment compared to the use of each alone 13. In the next step, the effects of cisplatin, Fisetin or their combined use on the process of apoptosis in cisplatin resistant A2780 cells were investigated.
One of the signs of apoptosis is the fragmentation of nuclear DNA. Therefore, DAPI fluorescent dye was used to investigate the fragmentation of nuclear DNA in treatment groups. The results showed that fragmentation of chromatin in cells occurred in the combined concentration of 0.1 μg/ml of cisplatin and 50 μg/ml of Fisetin. These effects were greater in the combined group than the group with either cisplatin and Fisetin use. The rate of apoptotic cells with broken chromatin was higher in this group. In a similar report by Chen et al on the combined effect of cisplatin and berberine on ovarian cancer cells, it was observed that A2780 ovarian cancer cells underwent fragmentation of the nuclei and the number of cells in combined treatments decreased, which is consistent with the results of this study 17.
Also, the staining method using acridine orange, propidium iodide, and Annexin/PI assay was applied in this study to determine the type of cell death. The results showed that at a concentration of 0.1 μg/ml of cisplatin and 50 μg/ml of Fisetin, the rate and number of apoptotic cells was higher than the use of each of them alone. Also, the results of Chen et al’s study showed that 17.2% of the cells underwent apoptosis in the combined treatment of cisplatin and berberine compared to the treatment of cisplatin in which only 5.7% of the cells had apoptosis. The results obtained in that study were consistent with the findings of the present study 17.
In addition, another study conducted by Gong et al showed that by increasing the dose of cayenne pepper in the combined treatment, more apoptosis occurs 13, which is consistent with the results of our study. However, in a study conducted by Kim et al in the combined treatment of alpha-lipoic acid with cisplatin in HEI-OC1 cells, the results showed a decrease in the effect of cisplatin in the combined treatment and the cells had no change in nucleus and in number, which is not consistent with the results of the present study 18.
In this study, in order to investigate the apoptosis pathway, the changes in genes expressions of Bax and BCL-2 were investigated. During apoptosis, Bax gene expression increases and BCL-2 gene expression decreases. The results of the present study showed that Bax pro-apoptotic gene expression decreased in the combined treatment compared to cisplatin treatment alone and BCL-2 anti-apoptotic gene expression decreased. However, in a study conducted by Kim et al on the protective and therapeutic effects of alpha-lipoic acid on the induction of cisplatin toxicity, a review of the results for Bax and BCL-2 genes showed that alpha-lipoic acid had no apoptotic induction effect on cisplatin. In combined treatment with cisplatin and alpha-lipoic acid, Bax pro-apoptotic gene expression decreases, and conversely, BCL-2 anti-apoptotic gene expression in combined treatment increases, which is consistent with the findings of the present study on Bax gene expression, and inconsistent concerning BCL-2 gene expression 18.
Sahu et al investigated the effect of Fisetin on reducing renal toxicity of cisplatin in mice through modulation of NF-κB activation and antioxidant defense. The results indicated a decrease in Bax pro-apoptotic gene expression in cisplatin and Fisetin in combined treatment compared to cisplatin treatment. Conversely, BCL-2 anti-apoptotic gene expression increased in combined treatment with cisplatin and Fisetin. The results indicate that a decrease in toxicity of cisplatin reduces the effects of nephrotoxicity in kidney cells 19 which is consistent with the results of this study. Considering the active role of caspases in apoptosis, based on the literature, the process of their activation is performed in different ways. In the intrinsic pathway, Bax and BCL-2 cause cytochrome c release from mitochondria membrane; cytochrome c activates caspase 9 and caspase 3, which have a significant role in apoptosis 20.
In the extrinsic pathway (Receptor pathway), a ligand of TNF family leads to activation of caspase 8 and subsequently caspase 3 21. The endoplasmic reticulum also activates caspase 12 through the release of Ca2+ and initiates apoptotic DNA fragmentation 22. The Golgi apparatus also activates caspase 2 through secretory pathway; it interferes with apoptosis through mitochondrial pathway as well as by reducing proteolytic activity and inducing fragmentation in the apoptotic process 23,24. In this study, gene expression in caspases 3 and 9 in combined treatments decreased compared to cisplatin treatment alone, but due to a greater increase in caspase 3 than in caspase 9, it can be concluded that apoptosis was induced by other pathways than the intrinsic pathway. A report by Tavsan and Kayali on the treatment of ovarian cancer cells by flavonoids showed that in flavonoid-treated cells, the increase of caspase 3 gene expression, decrease of caspase 9, and also increase of Reactive Oxygen Species (ROS) indicate that flavonoids cause apoptosis through extrinsic pathway 25, which is consistent with the results of this study.
Li et al observed that in apoptotic genes expression in combined treatment with cisplatin and crocin, cytochrome c gene expression increases. Due to the fact that cytochrome c activates caspases and eventually apoptosis through the mitochondrial pathway, it can be said that combined treatment of cisplatin with crocin increases apoptosis 26.
Conclusion :
Our study revealed reduction of ovarian cancer cell viability in cisplatin and Fisetin combined treatment compared to the use of each alone. Generally, the findings of this study indicated that Fisetin in combination with cisplatin can be considered effective in inhibiting the growth of cancer cells.
Acknowledgement :
The authors would like to acknowledge the contribution of Research Center for Animal Development Applied of Biology, Mashhad branch of Islamic Azad University, Mashhad, Iran in conducting the research.
Conflict of Interest :
There are no conflicts of interest.
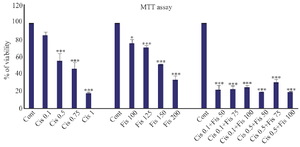
Figure 1. The toxicity of cisplatin, Fisetin, and combined application of these two compounds on A2780 ovarian cancer cells with different concentrations.
* Significance level at p<0.05, ** Significance level at p<0.01, *** Significance level at p<0.001.
|
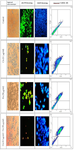
Figure 2. In control group during DAPI staining, the intact nucleus showed that the cells were healthy. In the cisplatin and Fisetin groups, A2780 cells underwent apoptosis after 24 hr of treatment and the cell nuclei were fragmented, and the rate was higher in the combined group. AO/PI staining: The number of apoptotic (Red) cells in the combined group is higher than the groups treated with either cisplatin or Fisetin (Magnification×10). The results of Annexin V-FITC/PI assay show that in the combined group, induction of apoptosis in cells under treatment was much more effective than the use of either Fisetin or cisplatin.
|
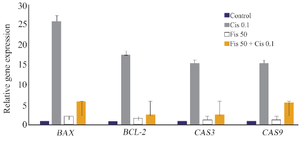
Figure 3. Changes in BCL-2, Bax, caspase 3, and caspase 9 gene expressions in experimental groups treated with Fisetin, cisplatin or a combination of both.
|
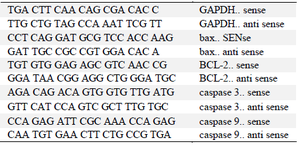
Table 1. Sequence of specialized primers
|
|