Designing a Strategy for pH Control to Improve CHO Cell Productivity in Bioreactor
-
Ahleboot, Zohreh
-
Biopharmaceutical Research Center, Aryogen Pharmed Inc., Alborz University of Medical Sciences, Karaj, Iran
-
Khorshidtalab, Mahdi
-
Biopharmaceutical Research Center, Aryogen Pharmed Inc., Alborz University of Medical Sciences, Karaj, Iran
-
Motahari, Paria
-
Biopharmaceutical Research Center, Aryogen Pharmed Inc., Alborz University of Medical Sciences, Karaj, Iran
-
Mahboudi, Rasoul
-
Biopharmaceutical Research Center, Aryogen Pharmed Inc., Alborz University of Medical Sciences, Karaj, Iran
-
Arjmand, Razieh
-
Biopharmaceutical Research Center, Aryogen Pharmed Inc., Alborz University of Medical Sciences, Karaj, Iran
-
Mokarizadeh, Aram
-
Biopharmaceutical Research Center, Aryogen Pharmed Inc., Alborz University of Medical Sciences, Karaj, Iran
-
 Maleknia, Shayan
Biopharmaceutical Research Center, Aryogen Pharmed Inc., Alborz University of Medical Sciences, Karaj, Iran, Tel: +98 26 336106480; Email:Maleknias@aryogen.com
Maleknia, Shayan
Biopharmaceutical Research Center, Aryogen Pharmed Inc., Alborz University of Medical Sciences, Karaj, Iran, Tel: +98 26 336106480; Email:Maleknias@aryogen.com
Abstract: Background: Drastic pH drop is a common consequence of scaling up a mammalian cell culture process, where it may affect the final performance of cell culture. Although CO2 sparging and base addition are used as common approaches for pH control, these strategies are not necessarily successful in large scale bioreactors due to their effect on osmolality and cell viability. Accordingly, a series of experiments were conducted using an IgG1 producing Chinese Hamster Ovary (CHO-S) cell culture in 30 L bioreactor to assess the efficiency of an alternative strategy in controlling culture pH.
Methods: Factors inducing partial pressure of CO2 and lactate accumulation (as the main factors altering culture pH) were assessed by Plackett-Burman design to identify the significant ones. As culture pH directly influences process productivity, protein titer was measured as the response variable. Subsequently, Central Composite Design (CCD) was employed to obtain a model for product titer prediction as a function of individual and interaction effects of significant variables.
Results: The results indicated that the major factor affecting pH is non-efficient CO2 removal. CO2 accumulation was found to be affected by an interaction between agitation speed and overlay air flow rate. Accordingly, after increasing the agitation speed and headspace aeration, the culture pH was successfully maintained in the range of 6.95-7.1, resulting in 51% increase in final product titer. Similar results were obtained during 250 L scale bioreactor culture, indicating the scalability of the approach.
Conclusion: The obtained results showed that pH fluctuations could be effectively controlled by optimizing CO2 stripping.
Introduction :
Monoclonal antibodies (mAbs) have become the major biopharmaceutical products over the recent decade 1. By taking advantage of their identical or similar post translational modifications to human, mammalian cells are considered as the main expression system for mAb production 2. In this regard, Chinese Hamster Ovary (CHO) cells are the most commonly used host system for expression of recombinant mAb 3. However, considering that CHO cell growth and protein production are highly influenced by the metabolic pathways, culture process parameters play a critical role in cell performance through the induction of certain metabolism pathways 4. Therefore, understanding the linkage between cell metabolism and process parameters is of particular importance for process optimization and scale up 5. pH, as one of the most critical process parameters in mammalian cell culture, has enough potential to modify cellular growth and specific productivity rate 6. Therefore, in almost all laboratory and large scale bioreactors, pH control system is automated to maintain pH at desired range by addition of acid or alkaline 7. However, controlling pH with acid or alkaline addition is often a challenge for pilot and large scale bioreactors since increase of bioreactor size can exacerbate the effect of high local pH excursions by increasing mixing time 8.
pH changes in the mammalian culture is mainly caused by lactate or carbon dioxide accumulation 9. Lactate is produced as a final product of incomplete glucose fermentation and accumulates during the later stages of the fed-batch culture which results in pH reduction 10. It has been shown that glucose and dissolved oxygen concentrations affect the lactate profile of mammalian cell culture process 11. Additionally, low CO2 removal rates can cause significant changes in pH 12. Agitation speed, sparge rate, bubble size, impeller position, and headspace aeration are considered as factors affecting pCO2 13. However, improving CO2 removal through increasing agitation speed is almost avoided mainly due to detrimental consequences of agitation induced shear stress 14. Moreover, due to the decreased surface area per volume in large scale bioreactors which result in a reduced aeration rate, sparging rate and bubble size are barely changed during scale up 15.
Previous studies indicated that the factors contribute to lactate production and CO2 accumulation should be considered during process scale up 16. Subsequently, development of a culture method with minimized lactate and CO2 accumulation not only improves manufacturing process but also increases process robustness 17. Different strategies can be used for optimizing process operating conditions to achieve optimum system performance. Design of Experiments (DOE) is a set of statistical approaches which is applied for modeling the bioprocess and offers several advantages over many traditional experimental designs 18. Accordingly, the obtained model can be used for optimization and prediction of the response under operational conditions 19.
The current research has focused on investigating the impact of critical parameters on cell culture pH and productivity by means of Plackett-Burman Design (PBD). Then, a Central Composite Design (CCD) was applied to study the interaction effects of overlay flow rate and agitation speed on the response variable. Additionally, the optimized levels were verified during scale up experiments. To the best of our knowledge, this is the first report of applying design of experiment as a statistical approach to control culture pH. This strategy takes advantage of studying the interaction and main effects of parameters.
Materials and Methods :
Cell line and culture media: A CHO-S (Thermo Fisher Scientific, USA, Catalog no: A11364) cell line expressing a 149 kDa anti-VEGF monoclonal antibody was obtained from Research Working Cell Bank (RWCB) of AryoGen Pharmed (Alborz, Iran). Cells were cultured in a chemically defined medium (GE Healthcare, Sweden) supplemented with 6 mM L-glutamine (Lonza, Belgium). Seed culture was prepared in a 500-ml baffled shake flask with an effective volume of 100 ml, incubated at 37°C with 5% CO2 and agitation speed of 75 RPM (Revolution Per Minute) (Infors LTD., UK) with 25 mm orbital diameter. The temperature was down-shifted to 32°C on day 5 of culture.
Cell culture analysis: Bioreactor samples were daily obtained and analyzed immediately. Viable cell density and viability were determined using 0.4% trypan blue exclusion-based method (Sigma, Canada). Osmolality was measured by Osmomat 3000 cryoscopic osmometer (Genotec, Germany). pCO2 was determined using Bioprofile 400 Analyzer (Nova Biomedical, USA). Glucose and lactate levels were assessed using a Biosen C-line lactate and glucose analyzer (EKF Diagnostics, Germany). Specific lactate production (qLac) was calculated according to equation 1 20. The expression level of target protein was measured at certain time points using an in-house enzyme linked immunosorbent assay (ELISA). Final concentration of product at the end of the fed-batch culture was determined by MAbPac Protein A affinity column (Thermo scientific, USA).
Equation 1: qlactate: ∆lactate × 90 ∆IV��C × ∆t
Δt is a time difference since the previous time point and IVCC (cell/ (ml* day)) is the integral cell density and is calculated by
Equation 2: IVCDn: VCD(tn)+VCD(tn-1)×(∆t)2+IVCD (t-1)
where tn and tn-1 (∆t) are the time at final and initial time points, respectively, and VCD (tn) and VCD (tn-1) are the cell density at final and initial time points, respectively.
Bioreactor operation: In this study, 30 L and 250 L stirred tank reactors (STR) (Biozeen, India) with three standard segment impellers and a frit sparger in 30 L bioreactor and a dual frit sparger system in 250 L were used. Bioreactor agitation speed was used to maintain the same power input per volume (P/V: 0.05) level between two scales, 145 RPM for 30 L bioreactor and 100 RPM for 250 L bioreactor. O2 flow was kept at 0.1 LPM (Liters Per Minute) and 3 LPM in 30 L and 250 L bioreactors, respectively. Overlay flow rate was examined between 5-15 LPM based on the experimental design. Vessel height to vessel diameter (H/D) ratio was taken as 1.5:1, while the impeller to vessel diameter (Di/Dt) was 0.34 in both bioreactors.
Bioreactor fed-batch culture: Bioreactors were inoculated to an initial working volume of 26 L and 178 L, respectively, at a target cell density of 0.65×106 cells/ml. Cultures were fed daily starting on day 2 by corresponding medium feed (2% feed A and 0.2% feed B). Glucose concentration was increased using 400 g/l glucose stock solution, once the value was below 1 g/l. Dissolved Oxygen (DO) was controlled at 60% of air saturation using pure oxygen sparging. A pH set point of 7.0 was adjusted with a dead-band of 0.1. Sodium bicarbonate buffer (0.5 M) was used to control pH at the set point according to Plackett-Burman experimental design.
Plackett-Burman experimental design: Plackett-Burman design was employed to investigate the potential influences of five operating parameters including glucose set-point, dissolved oxygen con centration set-point, gas flow rate (overlay), sodium bicarbonate buffer addition (to control pH) and agitation speed on the target mAb production (Buffer addition was selected as a categorical variable). In total, 18 saturated experiments (with three replicates at the center point) were designed using Minitab 18 (Minitab Inc., USA) to screen variables at two levels (Table 1). Values of operational parameters were chosen based on data obtained from preliminary experiments (DO set point, glucose set point, overlay flow rate, and agitation speed were adjusted between 40-60%, 1-3 g/l, 5-15 LPM, and 115-145 RPM). All experiments were carried out (duplicate) in 30 L STR bioreactors under conditions determined by the experimental design. The response variables were subjected to a regression analysis to find out the most important factors affecting productivity of the culture.
Statistical optimization of selected variables using central composite design: The two selected factors were subjected to RSM to evaluate their accurate relationship and to identify their optimal levels. Central composite design was applied to test 13 different process conditions in 30 L STR bioreactors. The ranges of values for the selected factors are shown in table 2. The center point was replicated three times to estimate the experimental error 21. The response data were analyzed to generate a quadratic (second order) polynomial model. The goodness of fit test was further measured through calculating correlation coefficient between the experimental and predicted values of the response variable.
Model validation: Cultures run in triplicate with the optimal values of the selected factors were used to verify the statistical model. The confirmatory experiments were then performed in large-scale bioreactor (three independent runs in 250 L STR bioreactor) and analyzed by Tukey's multiple comparison test. Constant impeller power per volume (P/V=0.05) was used as the scale up criterion. The rest of process parameters were kept un-altered, similar to values set for 30 L bioreactor.
Results and Discussion :
Screening of significant parameters by using PBD: Considering that a sudden decrease in pH in the acidic condition strongly affects the yield during the large scale production, optimization of cellular productivity necessitates control of different parameters affecting the culture pH. Accordingly, factors affecting lactate and CO2 accumulation were considered as the main parameters that could affect culture pH. Hence, 18 fed-batch cultures were run according to the design of experiment at different glucose and dissolved oxygen set points, along with different gas flow rates (overlay) and agitation speeds to investigate the effects of process parameters on culture pH and cellular productivity. pH control through buffer addition at desired pH set point was also studied to reveal its specific impact on final mAb expression level. It has been previously reported that air/N2 sparging can be used as a tool to control pH in large scale bioreactors 8. Nonetheless, such sparging strategy results in foam formation and an increased rate of contamination. Moreover, sparging-induced foam may alter cell metabolism and gas removal rate 22. Accordingly, air sparging was not involved in the Plackett-Burman screening design.
According to the PBD results, overlay flow rate and agitation speed were identified as the main factors significantly influencing mAb production (p=0.024 and p=0.001, respectively) (the variables with p≤0.05 are considered as significant factors) (Table 1). The main effects of process parameters are shown graphically using Pareto chart in figure 1. The positive coefficients (t-value) of significant parameters indicate that as the value increases, the dependent variable also (protein titer) increases (Figure 1), but a negative coefficient indicates that if variable increases, response variable decreases. Accordingly, increasing agitation speed and overlay flow rate (two significant parameters) resulted in elevated levels of response variable (mAb expression level).
The rest of parameters (buffer addition, glucose and DO set points) were identified insignificant (Table 1). It was previously reported that at low oxygen levels (low levels of DO set point), an enhanced formation of lactate leads to a drop in culture pH and productivity 23. As shown in table 3, DO set point in the examined ranges neither affected pH nor altered mAb expression level. This finding is in agreement with the results obtained by Restelli et al who did not find considerable differences in growth rate and final erythropoietin titer under a normoxic condition (normal oxygen concentrations) 24.
Glucose concentration can also be attributed to lactate accumulation. It has been shown that lactate formation slows down when glucose becomes limiting 25. Consequently, lactate concentration influences cellular specific productivity 26. In the present study, qLac was reported at levels of 1.7-3.3 pg/(cell×h) while the glucose set point varied between 1-3 g/l (Table 3). However, there was no correlation between lactate accumulation within the observed range and final resulted titer (p>0.05). This finding is consistent with the result of a previously published study reported that lactate accumulation is not capable of altering pH dramatically which is attributed to antibody production 20.
Although alkaline or acid addition and controlled CO2 sparging are typically general solutions for pH control in bioreactors 8, the analysis of PBD revealed that controlling pH fluctuations by buffer addition had no significant influence on the response variable (mAb titer) (Table 3). Compared to the untreated control culture, buffer addition to control pH resulted in a substantial increase in osmolality (experiment run numbers 7 and 9) along with a dramatic reduction of 51±3% in the cell viability. Even though after buffer addition culture pH was kept in the desired range, the final expression level of mAb was not improved (Table 1). Taken together, pH control by buffer addition was not efficient to enhance culture productivity either due to elevated levels of osmolality or loss of viability. Accordingly, Zhou et al reported the adverse effect of high osmolality on antibody-fusion protein production 27. In addition, it has been reported that pH control with alkaline addition can lead to high local pH excursions when mixing is insufficient 28.
Table 3 exhibits the Maximum Viable Cell Concentration (MVCC), cell viability, titer value, qLac, pCO2 and pH profile for each PBD experiment. As the results show, the experimental runs with higher productivities are correlated with higher average pH value and lower pCO2 levels. Nonetheless, there was not a statistically significant difference in MVCC among conditions [One-way analysis of variance (ANOVA) was applied to determine the statistical significance (p=0.05)]. According to the results, pCO2 was found as the main parameter influencing culture pH where it is controlled by agitation speed and overlay flow rate.
Taken together, it can be hypothesized that control of pCO2 is not only favorable but also urgent for an effective control of culture pH. Even though the negative consequences of elevated levels of pCO2 have clearly been explained 29, an effective and practically possible control strategy has not been introduced yet for most of the industrial processes 30. Considering that CO2 accumulation is affected by two main parameters (agitation speed and overlay air flow rate), effect of these parameters were tested by CCD to find the optimal level for each parameter.
Model building and statistical analysis based on CCD: Semi-large scale fed-batch cultures were carried out in 30 L bioreactor while agitation speed and overlay air flow rate were separately and simultaneously changed as variables to determine their single and interaction effects on the antibody titer using a central composite design. The expression levels of antibody which was measured for 13 cultures are summarized in table 2. The expression level of experiments ranged from 950 to 1850 mg/l, indicating the importance of identifying an optimal process condition. In order to evaluate the accuracy and reproducibility of the model, experiments were performed with center point values replicated five times (130 RPM and 110 LPM).
According to the CCD results, lower levels of overlay flow rate and agitation speed (run numbers 6 and 7) resulted in lower culture pH (6.83±0.10 and 6.88±0.10, respectively) which then led to reduced productivity (950 and 1165 mg/l, respectively) (Table 2). To further determine the correlation of the expressed IgG levels and the corresponding variables, their relationship was modeled by the following equation.
Equation 3:
Y=-11272+327.4 A+157.51 B-11.685 A×A-0.5232 B×B-0.567A×B
Y is the mAb titer (mg/l), and A and B are overlay flow rate (LPM) and agitation speed (RPM), respectively. The significance level of the equation was evaluated by F‑test and the analysis of variance for response surface quadratic second order model (Table 4). According to the results, the square of agitation speed (B2) and overlay flow rate (A2) and the interaction term (AB) were statistically significant. The calculated F-value of agitation speed (1438.94) and overlay flow rate (270.05) and analysis of variance for selected parameters showed that the model was significant (p≤0.05). Furthermore, the regression model for mAb titer showed a significant value of the coefficient of determination (0.9975), a reasonable agreement for the predicted R2 (0.9887) with the adjusted R2 (0.9957). Additionally, the lack-of-fit value of 0.34 indicated the validity of the model with respect to the pure error.
The response surface and 2-D contour plots which were created using equation 3 are shown in figure 2. The elliptical contour plot indicated the significant interactions between the variables 31. Figure 2 represents response surface plot of two independent variables. It can be seen that maximum mAb titer was obtained when overlay flow rate was kept at its middle level (10 LPM). Higher values of overlay flow rate led to an increase in culture medium concentration as a result of higher evaporation which induced higher osmolality of culture medium above 500 mOsm/kg. Sudden decrease in cell viability occurred at elevated osmolality. This might be the cause of reduced mAb titer at very high overlay flow rates (15 LPM). Otherwise, lower flow rates were not effective in both pH control and CO2 removal indicating that there is an optimal level of headspace aeration for cell culture operation. Similarly, the role of surface aeration in CO2 clearance from the headspace was reported elsewhere 32. Moreover, the mAb titer was enhanced by increasing the agitation speed from 110 to 150 RPM (Figure 2). A comparative analysis of lactate, pCO2 and pH in the cultures grown at low agitation speed and the cultures agitated at high speed based on the estimation of the antibody titer revealed a different pH profile with the change in CO2 accumulations but not in residual lactate concentrations (Figure 3). Therefore, it seems that the higher agitation speed was favorable for the CO2 removal and pH control. These results are in contrast with findings of Shetty who reported the minimal effect of varying agitation rates on NS0 cell growth and productivity 33.
The CCD revealed that elevated levels of pCO2 could be effectively controlled through interaction effect of overlay air flow rate and agitation speed in large scale cell culture. Hence, the control of pH in a desired range can be obtained by optimizing the overlay flow rate and agitation speed simultaneously.
Model validation: The point prediction feature of the RSM was used to calculate the optimal value of each factor (Figure 4). Three independent experiments were performed under the optimal condition to verify the model in which the agitation speed was 145 RPM and overlay air flow rate was 10.5 LPM. The appropriateness of the response model was justified by the strong correlation between the predicted and experimental values (p>0.05). Following the optimization for process condition, a 51% increase in final protein titer was achieved.
Scale up study: With the optimized parameters, the cell culture was scaled up to 250 L bioreactor while a constant impeller power per volume (P/V) was considered as the scale up criterion. According to the results of two-way analysis of variance, there were no significant differences in maximum cell density, viability, mAb titer, pH, pCO2, and lactate specific production between 30 L and 250 L bioreactors, indicating the reliability and robustness of the method (Figure 5). These results confirmed that the interaction effect of increasing headspace aeration and agitation speed was effective to improve the mAb production in pilot 250 L scale.
A preliminary study concerning the effect of a single factor (overlay air flow rate) on mAb titer revealed that increasing overlay flow rate singly is not enough for removal of CO2 in large scale bioreactors (data not shown). This observation may be attributed to the lower surface area to volume ratio in large scale bioreactors where it resulted in longer gas retention time 34. Therefore, increasing the flow rate of surface aeration (overlay flow rate) singly was not considered as an efficient approach for CO2 stripping. However, this problem was overcome by combining the optimal level of agitation speed and surface aeration which resulted in effective pH maintenance in range of 6.9-7.1 (Figure 5D).
Conclusion :
pH is one of the most important parameters affecting productivity particularly in industrial manufacturing process. This is highly due to the fact that the highest specific growth rate and the maximum cell specific productivity are often achieved only over a narrow pH range. Accordingly, in the present study, the purpose was finding an efficient method to control pH through accelerating CO2 removal. Optimization of effective parameters by CCD provided a good approximation to interaction effect of agitation speed and overlay flow rate for controlling pH and improving productivity. Even though the impact of agitation rate on the efficiency of CO2 removal has been reported previously, only few studies investigated the effectiveness of overlay flow rate on CO2 stripping. The obtained results highlighted the positive interaction effects of these two parameters in controlling culture pH simultaneously followed by significant increases in CO2 removal and mAb expression level (by about 51%). This strategy can be considered as an effective and easily applicable method for controlling pH and improving productivity in industrial manufacturing processes.
Acknowledgement :
This work was performed in Biopharmaceutical Research Center of AryoGen Pharmed Inc., under ethical supervision of Alborz University of Medical Sciences with ethical code of IR.ABZUMS.REC.1398.038.
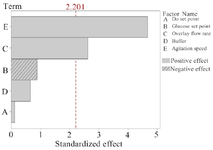
Figure 1. Pareto chart of standardized effect. The chart demonstrates agitation speed and overlay flow rate are the variables with significant effects on the response variable (mAb titer). The length of each bar corresponds to the standardized effect or interaction and the vertical line indicates the significant effect at a confidence interval of 95%.
|
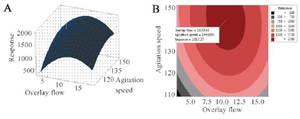
Figure 2. A) Three-dimensional and B) Contour graphs showing the effect of agitation speed (RPM) and overlay flow rate (LPM) on mAb production (mg/l). The plots showed that increasing the agitation speed to ̴150 RPM and overlay flow rate to ̴10 LPM resulted in optimal level of response (Protein titer).
|
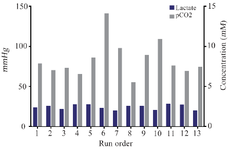
Figure 3. Influence of selected parameters on lactate and CO2 production in CCD experimental design. Lactate and CO2 concentrations during 13 runs of CCD experiments indicated that lactate accumulation is not different between cultures. Therefore, agitation speed and overlay flow rate control pH by changing pCO2 in the culture.
|
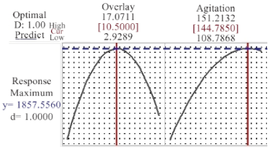
Figure 4. Prediction values of variables and final mAb titer using response optimizer module of Minitab. Agitation speed of 144.78 RPM and overlay flow rate of 10.5 LPM are predicted as optimal values for reaching the maximum mAb titer of 1857.5 mg/l.
|
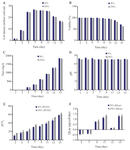
Figure 5. Culture performance in 30 L and 250 L bioreactors. A) MVCC (106 cells/ml), B) viability (%), (c) titer (mg/L), D) offline pH, E) pCO2 (mmHg), and F) lactate specific production (qLac) (pg/(cell×h)) during 15 days of culture. Bars represent standard deviation. The scalability of the predicted values are shown in the similar patterns between 30 L and 250 L scale bioreactors (n=3; mean±SD).
|
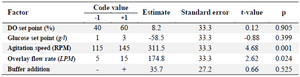
Table 1. Experimental range, estimate, standard error, t-value and p-value for the factors screened in the PBD
|
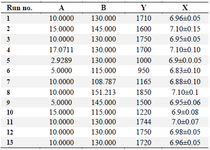
Table 2. Thirteen trials of the CCD matrix for (A) overlay air flow rate (LPM) and (B) agitation speed (RPM) with the response (Y) (mAb titer) (mg/l) and average pH of 15 days of culture (X)
|
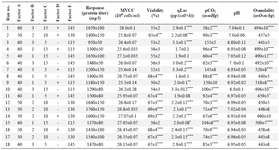
Table 3. Experiments using Placket-Burman design for process variables contribution to mAb production in coded units. A) DO set point (%), B) glucose set point (g/l), C) overlay air flow rate (LPM), D) buffer addition, and E) agitation speed (RPM). The results of protein titer (mg/l), viability (%), pCO2 (mmHg), and osmolality (mOsm/kg) at the end of the culture are presented. qLac (pg/(cell×h)) and pH are the average values of whole process (Samples were taken daily for 15 days)
Asterisks indicate levels of statistically significant differences between experimental runs and control condition (Glucose set point, DO set point, overlay flow rate, and agitation speed were adjusted to 1 g/l, 50%, 5 LPM, and 115 RPM, respectively). (*p<0.05, **p<0.01, ***p<0.001 and ****p<0.0001).
|
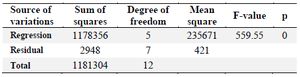
Table 4. ANOVA results of the response surface quadratic model for mAb production
|
|