Developmental Toxicity of the Neural Tube Induced by Titanium Dioxide Nanoparticles in Mouse Embryos
-
Mohamadzadeh, Nahid
-
Department of Anatomical Sciences, Faculty of Medicine, Urmia University of Medical Sciences, Urmia, Iran
-
 Zirak Javanmard, Masoumeh
Department of Anatomical Sciences, Faculty of Medicine, Urmia University of Medical Sciences, Urmia, Iran, Tel: +98 44 33486216, E-mail: zirakjavanmard.m@umsu.ac.ir
Zirak Javanmard, Masoumeh
Department of Anatomical Sciences, Faculty of Medicine, Urmia University of Medical Sciences, Urmia, Iran, Tel: +98 44 33486216, E-mail: zirakjavanmard.m@umsu.ac.ir
-
Karimipour, Mojtaba
-
Department of Anatomical Sciences, Faculty of Medicine, Urmia University of Medical Sciences, Urmia, Iran
-
Farjah, Gholamhosain
-
Department of Anatomical Sciences, Faculty of Medicine, Urmia University of Medical Sciences, Urmia, Iran
Abstract: Background: This study investigated the potential effects of Titanium dioxide nanoparticles (Tio2NPs) followed by maternal gavage on fetal development and neural tube formation during pregnancy in mice.
Methods: Thirty pregnant mice were randomly divided into five main study groups including the untreated control and 4 experimental groups (n=6 per group). The control group was treated with normal saline and the experimental groups were orally treated with doses of 30, 150, 300, and 500 mg/kg Body Weight (BW) of Tio2NPs during pregnancy. On gestational day 16 and 19 (n=3 per group), pregnant mice were euthanized and then examined for neural tube defects and compared with control. Serial transverse sections were prepared in both cranial region and in lumbar region of spinal cord.
Results: Treatment with Tio2NPs resulted in low fetal weight and short length, dilation of lateral ventricle, thinning of cerebral cortex and spinal cord, spina bifida occulta and an increase in the number of apoptotic neurons in exposed embryos at doses of 300 and 500 mg/kg (p<0.05).
Conclusion: It seems that exposure to nanoparticles of Tio2 during pregnancy induces growth retardation and for the first time, teratogenicity of this nanomaterial in neural tube development and induction of defects such as spinal bifida, reduction in cortical thickness and dilatation of lateral ventricles were verified which can be related to incidence of apoptosis in central nervous system.
Introduction :
During neurulation, the neuroepithelium elevates at both sides of Medial Hinge Point (MHP); it bends on the sides of dorsolateral walls and completes the fusion forming neural tube 1. Recent developments in the field of neurulation indicate that during elevation, the rate of cell proliferation increases and the neuroepithelial cells migrate ventro-dorsally; therefore, it seems that these two factors cause the fusion process 2. Some genetic, environmental and nutritional factors can affect the normal developmental process of neural tube formation and cause the incidence of Neural Tube Defects (NTDs) such as spina bifida which is one of the most common forms of human congenital defects 1. In contrast to other developmental disorders, closure of neural tube occurs in the first month of embryonic period, and many pregnant women do not avoid harmful factors due to unawareness of their pregnancy 3.
An important consideration is the wide use of particles in industry, food packing, medical application, water and air 4-6. Titanium dioxide Nanoparticles (Tio2NPs) are in the list of the five top nanoparticles with highest consumption 7. The broad range of application, potential long term tissue accumulation and induction of tissue damages seem to raise concern with regard to its potential embryonic toxicity 8. An expanding commercialization, massive production and wide use of personal care products of nanoparticles resulted in a high potential adverse health risk. People are exposed to NPs through various routs; sunscreen and toothpaste significantly contribute to Tio2 dermal exposure. The estimated personal dermal exposure is 8 to 21.4 mg per day, while 10% of toothpaste is ingested daily 9. Titanium dioxide (Tio2) as a part of the modern diet will essentially increase their oral intake worldwide. Tio2NPs are widely used as a food color in confectionaries in sauces, fondant and icing. The average daily exposure to Tio2 for older adults is 0.5-1.1 mg and for children is 1.4-3.2 mg per kg body weight 10. While the risk of particle inhalation received much attention, gaps of knowledge exist regarding possible adverse health effects through digestion 11.
By any route of exposure, it enters the blood circulation and has the ability to cross the blood-barriers of testis, placenta and brain 12-14. There are very few experiments that have reported about the teratogenicity of NPs. The injection of iron oxide NPs (10 mg/kg) during gestational days (GD 9-GD16) increased fetal death and induced external abnormality in limb, tail and ribs 15. Injection of Tio2 (35 nm size) to pregnant mice resulted in growth retardation, fusion of ribs, vertebrae, and phalanges 13. Another study has reported that oral single dose of Tio2 (51-65 nm, 100 and 1000 mg/kg) on GD10 induced limb and tail deformity and exencephaly, without weight loss of mice fetuses 16. The most relevant reports to our research was oral administration of Tio2 (diameter of 6.5 nm, dose of 50 and 100 mg/kg, GD 0-GD17) to pregnant ICR mice which induced growth retardation, skeletal malformations, exencephaly and spina bifida as a reduction of ossification in sacral arches by macroscopic evaluation using Menegola’s method 17.
The hazard to pregnant women and the possibility of NPs reaching the developing fetus are of particular concern. Due to the lack of protection mechanism, embryos are more susceptible to environmental exposures 18. The mouse is a common mammalian model for embryonic toxicity study. Despite the rapid introduction of nanomaterials to markets, their safety has not yet been well established. The CNS is the potential susceptible target of NPs, but studies on this aspect are limited. Therefore, in this study, different concentrations of Tio2 were used in experimental groups and it was hypothesized that higher doses of Tio2 would result in more toxicity in embryonic development with neural tube defects in both cephalic and caudal parts.
Materials and Methods :
Thirty-five female albino mice (20±5 g) were purchased from the Animal Center of Urmia University of Medical Sciences (Iran). Having been ensured the mice were non-pregnant, they were acclimated to the environment for 10 days, then were kept in cages at a ratio of 3:2 (Female: male) under a controlled environment at 22.00°C and a 12-hr light-dark cycle for coupling. The next morning, the mice were examined for vaginal plug; the ones with a vaginal plug were isolated and it was the sign of day 0 of pregnancy.
Pregnant mice were housed individually and randomly divided into five main study groups including the untreated control and experimental (n=6 per group). The control group was treated with normal saline, whereas the experimental groups were treated with 30, 150, 300, and 500 mg/kg BW of nano-Tio2 17. Each experimental group was further divided into two subgroups based on the day of sacrifice (Embryonic days E16 and E19) with 3 pregnant mice in each.
Animals received 30, 150, 300 and 500 mg/kg BW of nano-Tio2 (99% anatase, crystal structure) with size of 10-25 nm in diameter (Sigma-Aldrich Japan., Tokyo) by oral gavage from gestational days 1 to, 15 and 18 in an isolated animal room. On gestational days 16 and 19, pregnant mice were anesthetized lightly with chloroform and euthanized. The uterus was quickly removed, and then 16-day and 19-day-old fetuses were removed from uterus and the weight and crown-rump length were measured. All experiments were performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and approved by Ethics Committee of the Urmia University of Medical Sciences (Ir.UMSU. rec.1395.29).
Macroscopic examination: The uterus was opened to remove the fetuses and to record the number of absorbed or dead fetuses. All 16 and 19 day old fetuses in control and experimental groups were examined macroscopically under stereomicroscope for external morphological malformations.
Histopathological examination: For microscopic study, three fetuses in each litter were fixed in 4% paraformaldehyde. For further histological analysis, the paraffin cross sections of the developing cerebral cortex and lumbar spinal cord sections were cut at 5 μm and were stained with hematoxylin-eosin and TUNEL assay, respectively in order to follow up the toxic effect of Tio2 in the neural tube tissue and apoptotic cells.
Morphometric analysis: Cross-sectional morphometric measurements were performed on each fetal telencephalon and spinal cord. In cerebral region, the measurement was performed in each fetal brain, identified in cross section by the presence of lateral ventricles, and third and fourth ventricles which were arranged from anterior to posterior part. The anteroposterior diameter of right lateral ventricle was drawn, and then the midpoint of diameter was considered for measuring lateral ventricular thickness. Also, the environment of right lateral ventricle was measured using Motic software. The criterion of spinal cord region was the presence of kidneys. In this level, the thickness of spinal cord was measured in posterior commissure. All measurements were performed and slides were screened at a 10× magnification.
Immunohistochemical staining: Cell quantification was performed on transverse sections of cortex and spinal cord of at least three animals in each group. To investigate whether Tio2NPs could decrease and/or increase the number of apoptotic neurons, immunohistochemical staining was performed via terminal deoxynucleotidyl Transferase dUTP Nick End Labeling (TUNEL) assay (In situ Cell Death Kit, POD, Mannheim, Germany) detecting DNA fragments according to the manufacturer’s instructions. Slides were incubated with 0.1% Triton ×100 and exposed to terminal deoxynucleotidyl transferase and then peroxidase-conjugated antidigoxigenin antibody. For background staining, Mayer's hematoxylin was used. For quantification of apoptotic cells, TUNEL-stained sections were imaged at a 40× magnification. The number of TUNEL- positive cells was counted and registered.
Statistical analysis: Statistical analyses were performed using SPSS version 16. One-way analysis of Variance (ANOVA) was run for comparison across groups. Statistical significance was set at p≤0.05.
Results :
Pregnant mice were exposed to different doses of Tio2 (30, 150, 300 or 500 mg/kg) at selected time points of embryonic development (E16 or E19) and were observed macroscopically and microscopically. Only one craniofacial malformation and one absorbed fetus were observed at dose of 500 mg/kg on embryonic day 19.
Fetal growth: Pregnant mice were euthanized on gestational days 16 and 19, and then fetal body weight and crown-rump length were measured. These two factors significantly decreased in Tio2 exposed groups expect the low dose treated group (30 mg/kg) compared to the untreated control group. With increasing dose of Tio2 (Figure 1), weight and height loss was markedly observed.
Lateral ventricular dilatation: In order to identify possible damages in the cortical region, morphometric analysis was used. In the cortex of the control group with normal development, the molecular, external granular and external pyramidal layers were recognized separately. Quantitative measurements demonstrated that the cortical thickness of Tio2 exposed groups was mildly decreased at doses of 30 and 150 mg/kg, but reduction rate was significant at two high doses. The same result was obtained for morphometric parameters of right lateral ventricle at doses of 300 and 500 mg/kg (Figure 2).
Spinal cord development: The microscopic observations of transverse sections stained with H&E showed that Tio2 affects the spinal cord development and formation of vertebrae. The thickness of spinal cord at the posterior commissure line decreased significantly in the 500 mg/kg exposed group as compared to the control. The vertebral column development showed the presence of spina bifida occulta in all exposed groups which was significantly higher in the 300 and 500 mg/kg treated groups. In the skeletal part of vertebral column, the body, pedicles, and transverse processes were formed and both laminas were fused together; spinous process started on embryonic day 16 (E16), and was well formed on E19. The ossification islands were apparent in all parts of control vertebrae, but in nano scale Tio2 exposed groups, the ossification centers were absent or decreased. The incomplete formation of laminas or presence of spina bifida occulta was the most important defect in the process of vertebral column formation in treated groups (Figure 3).
Cortical and spinal cord apoptosis: Using TUNEL immunohistochemistry, apoptosis was analyzed in the embryonic neuroepithelium surrounding the lateral ventricle and spinal cord (Figure 4). In the cortical region, most cell deaths occurred in the outer layers of cortex. Cortical and spinal cord cells underwent apoptosis in response to Tio2 even at low and moderate doses of nanoparticles. Finally, there was significant difference between 300 or 500 mg/kg groups compared to control on E16 and E19.
Discussion :
In this study, growth retardation, decreased Body Weight (BW) and fetal crown-rump length were observed in Tio2 exposed group, which became more severe with increasing doses. These results are in contrast to previous findings demonstrating that oral administration of titanium (21 nm, anatase/rutile) to rats during GD6-GD19 at a high dose (1000 mg/kg) would not result in significant weight loss in fetuses 20. This difference in developmental results is related to variability in animal species, chemical composition, particle size and duration of exposure 21. However, the findings are consistent with the previous study which demonstrated that gavage of a dose of 100 mg/kg of Tio2NPs caused reduced fetal weight and length as markers of teratogenicity 17. Another study has also reported that intravenous exposure to Tio2 (35 nm) induced developmental retardation as smaller fetuses developed in mice 22. According to a new published review, after parental exposure, Tio2NPs accumulate in placenta and cause impairment in nutrients conduction which in turn results in fetal growth retardation 23. Inhaled Tio2 nanoparticles increased microvascular oxidative stress up to 60% and resulted in impairment of muscle arteriole dilation 24. In addition, impaired coronary arteriolar endothelium-dependent dilation and increased ROS of vessels have been reported 25. Placenta is one of the target organs for this particle, thus some nano particles can affect the placental blood flow and substance transport. They induce anorexia and necrosis of uterus which probably results in reduced size of fetuses 26. It seems that developmental retardation may be related to impairment of placental capillaries in the villi, uterine vascular dysfunction and greater inflammatory response of vessels 27.
Incidence of spina bifida occulta and failure of closure of vertebral arch as well as decreased ossification centers of vertebrae at high doses (300 and 500 mg/kg) of exposure were seen in this study. Maternal exposure to Tio2 during gestational days revealed increased titanium concentrations in maternal and fetal serum and resulted in reduction in the calcium and zinc contents, reduced ossification, rib and sternum absence 17. In vivo experiment demonstrated that two weeks of mothers’ exposure to Tio2 degenerated chondrocytes and mesenchymal cells of forelimbs in mouse embryos 28. It has been reported that the aluminum nanoparticle through parental nutrition can impair bone mineralization and induce neurobiological delay 29. Inhibition of osteogenesis in cell cultures, by induction of YAP signaling pathway after exposure to 10 ppm titanium is another in vitro report 30. Changes and impairment of placental blood flow may decrease the permeability of Ca+2 in the placenta. Tio2 interferes with calcium metabolism in embryo and affects the ossification. These particles damage osteoblasts and increase osteoclast activity, which in turn would decrease the rate of ossification. The maternal calcium and zinc reduction may be an important cause of Tio2 induced growth retardation 17.
Thinning of the cortical plate and suppression of mitosis (Figure 2) in fetal neuroepithelial tissue of the exposed mice were the additional evidence that confirmed damage to the fetal cortical plate cells in the present experiment. The data of five measurements/ groups obtained here on the fetal lateral ventricles demonstrate that the administration of Tio2 NPs to pregnant mothers affects the central nervous system of offspring. Similar to our finding, exposure toTio2NPS during pregnancy and lactation periods reduced cerebral thickness, number of neurons and induced edema in cerebellum and cerebral areas by the reduction of Rac1 and Cdc42 that are involved in the development of axon and dendrite 31. Findings have showed that Tio2 NPs accumulate in the brain and lead to hippocampal cells apoptosis, glial cells proliferation and induction of oxidative stress 32. Oxidative stress and apoptosis are the main developmental toxicants which disrupt the cellular and molecular processes of physiological development and generate embryonic malformations 33. Another hypothesis suggests inhibition of the cell division following exposure to Tio2, which could result from a disturbance of the enzymes involved in DNA replication and cell cycle 34. It has been recently reported that after exposure to Tio2 NPs, these particles accumulate in ventricles, cortex and cerebellum and induce an increase of necrotic cells in nervous tissue. A significant decrease in neuroblasts and an increase in apoptotic neural cells were observed in cortical cell cultures followed by Tio2 treatment at the dose of 20 µg/ml 35. Immunohistochemical staining revealed ROS production and lipid peroxidation of cerebral cortex 36.
Conclusion :
There is only one previous report which indicated incidence of spina bifida after exposure to Tio2 NPs. The histological assessment of spina bifida, ventricles’ enlargement and cortical thinning is the first report on teratogenicity of Tio2. Embryonic cell death and delay in growth may be responsible for the observed abnormalities. For this reason, more molecular researches are recommended to investigate the mechanism of neural tube defects.
Acknowledgement :
This study was supported by Vice-Chancellor for Research of Urmia University of Medical Sciences.
Conflict of Interest :
The authors notify that they have no conflicts of interest.
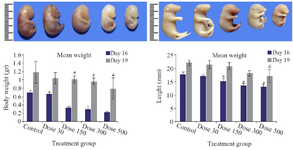
Figure 1. Macroscopic examination of fetuses on E16 (Upper row) and E19 (Lower row) revealed stunted growth including body weight loss and decrease of length in Tio2 NPs exposed groups. Size reduction was significant at doses of 300 and 500 mg/kg of Tio2 NPs compared with the control group, p<0.05. # comparison with control on E16 and * E19.
|
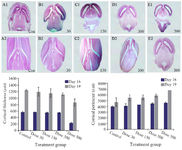
Figure 2. Reduction in cortical thickness and dilatation of lateral ventricles in the control and Tio2-NPs exposed embryos on E16 (A1-E1) and E19 (A2-E2). Heads of control and exposure groups were transversely sectioned and stained with H&E. Decreased cortical thickness and lateral ventricular dilatation after nanoparticle exposure increased significantly (p<0.05) at higher doses (300 and 500 mg/kg) compared with control. Data are presented as mean±SD. # comparison with control on E16 and * E19.
|
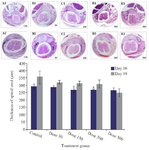
Figure 3. Transverse section of spinal cord and formation of vertebra in the control and Tio2-NPs exposed embryos on E16 (A1-E1) and E19 (A2-E2). Microscopic sections showing complete formation of vertebra in control group, fusion of Right Lamina (RL) and Left Lamina (LL) and formation of Spinous Process (SP). The red curve represents the distance between the laminas. Data are presented as mean±SD. # Comparison with control on E16 and * E19.
|
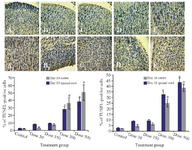
Figure 4. Photomicrographs of TUNEL-stained sections of fetuses in cortex (A1-E1) and spinal cord (A2-E2) on E19. The apoptotic cells were predominant in Tio2 NPs groups compared to the control group. The number of TUNEL-positive cells (Red arrow) was significantly higher in the 300 and 500 mg/kg groups than in the control group, # comparison with control on E16 and * E19.
|
|