Production and Characterization of Mouse Monoclonal Antibodies Recognizing Multiple Subclasses of Human IgG
-
Hajighasemi, Fatemeh
-
Department of Immunology, School of Public Health, Tehran University of Medical Sciences , Tehran, Iran
-
Department of Immunology, School of Medicine, Shahed University , Tehran, Iran
-
 Shokri, Fazel
Ph.D., Monoclonal Antibody Research Center, Avicenna Research Institute, ACECR, Tehran, Iran, P.O. Box: 6446-14133, Tel: +98 21 22431945, Fax: +98 21 22432021, E-mail:fazshok@yahoo.com
Shokri, Fazel
Ph.D., Monoclonal Antibody Research Center, Avicenna Research Institute, ACECR, Tehran, Iran, P.O. Box: 6446-14133, Tel: +98 21 22431945, Fax: +98 21 22432021, E-mail:fazshok@yahoo.com
-
Department of Immunology, School of Public Health, Tehran University of Medical Sciences , Tehran, Iran
-
Monoclonal Antibody Research Center, Avicenna Research Institute, ACECR , Tehran, Iran
Abstract: Different IgG subclass profiles are produced in response to different antigenic stimuli in a variety of diseases. IgG subclass levels may reflect disease severity. Quantification of IgG subclasses depends on the availability of specific Monoclonal antibodies (MAbs). In the present study seven hybridoma clones producing MAbs reactive with multiple subclasses of human IgG were established. Splenocytes from Balb/c mice immunized with Fc fractions of human IgG1 or IgG2 myeloma proteins were fused with mouse myeloma cells. Fused cells were selected and cloned by limiting dilution assay. Antibody secreting cells were screened by Enzyme-linked immunosorbent assay (ELISA) and the specificity of secreted MAbs was further analyzed, using a panel of purified human myeloma paraproteins of different IgG subclasses by ELISA and immunoblotting. Cross-reactivity to immunoglobulins (Igs) of other species was studied by indirect ELISA using serum samples collected from 9 animals. The MAbs were found to react with triple IgG subclasses, including IgG1,2,4 (n=4) and IgG1,2,3 (n=3). Immunoblotting studies revealed recognition of linear (n=4) or conformational (n=3) epitopes by these MAbs. The most abundant cross-reactivity (71.4%) was observed with monkey Ig while no cross-reactivity was detected with hen and cat sera. The MAbs mostly displayed a restricted pattern of cross-reactivity and one of them did not bind to any of the animal sera tested. The affinity constant of 3 MAbs was measured by ELISA. Based on the data obtained from this study, mouse MAbs reactive with multiple human IgG subclasses are directed to a variety of immunogenic epitopes, mostly shared with IgG of other species. These MAbs are valuable tools for purification of non-reactive IgG subclasses through negative affinity chromatography. These MAbs could also provide an opportunity for epitope mapping of the Fc region of IgG, as well as serological phylogenetic studies.
Introduction :
IgG, the most abundant Ig present in human serum and extravascular fluids, is subdivided in to four subclasses (IgG1-4) based on antigenic differences between their heavy chain constant regions (1). Each subclass has different biological characteristics (2) and a particular profile of effector functions (3). Immunoglobulin subclasses are differentially regulated in response to different antigenic stimuli (4, 5). Thus different patterns of IgG subclasses (6 - 8) have been reported to be produced in a variety of diseases. Moreover, there is a relationship between IgG subclass levels and severity of diseases (9, 10). Therefore IgG subclasses have a great diagnostic and prognostic value (11). Analysis of the IgG subclass response to different antigens of a microorganism may reveal the profile of T helper associated cytokine response (12) and hence is helpful for designing a potential vaccine (13). Quantification of IgG subclass levels depends on the availability of specific MAbs. Assay sensitivity could be improved by application of high affinity MAbs recognizing appropriate epitopes (14).
Since human IgG subclasses are highly homologous with almost 95% aminoacid sequence identity (15), some human IgG subclass restricted mouse MAbs may recognize epitopes shared by multiple IgG subclasses. Such MAbs are valuable tools for epitope mapping of human IgG isotype and may recognize isotypic or isoallotypic markers within IgG subclasses.The aim of the present study is production and characterization of MAbs with specificity for multiple IgG subclasses.
Materials and Methods :
Preparation of purified human IgG subclasses:A panel of 27 different purified human IgG myeloma proteins of known IgG subclasses and light chain types was employed in this study. These myeloma proteins, obtained from patients with multiple myeloma, were either purified by Diethyl amino ethyl (DEAE) cellulose (Whatmann, UK) chromatography or by affinity chromatography using Staphylococcal protein A (SPA) or Streptococcal protein G (SPG) Sepharose 4B (Pharmacia, Sweden). The heavy chain and light chain isotypes and subclasses of myelomas were identified using specific mouse monoclonal antibodies including: AF6 (IgM), 8a4 (IgG), 2D7 (IgA), JA11 (IgD), C4 (?), 6el (?), JL512 (IgG1), GOM2 (IgG2), ZG4 (IgG3) and RJ4 (IgG4), kindly provided by Professor R. Jefferis and Dr. M. Goodal (Dept. of Immunology, University of Birmingham, UK).
Polyclonal IgG was isolated from normal serum with SPG-Sepharose and polyclonal IgG3 was isolated, as break-through fraction, from polyclonal IgG by SPA-Sepharose column. Fc and Fab and F (ab?) 2 fragments were produced from several purified human myeloma proteins of each of the IgG subclasses, by pepsin and papain digestion (16). Digested fragments were isolated by affinity chromatography with SPA-Sepharose column.
Animal sera:Sera from human and nine animals were prepared from their clotted blood. The animal species used in this study were chicken, rabbit, guinea pig, cat, dog, sheep, goat, horse and monkey. The human serum was used as a control.
Production and selection of hybridomas:Balb/c mice (8-12 weeks of age) were immunized with four intraperitoneal injections of Fc fragments of human IgG1 or IgG2 myeloma proteins emulsified in Freund?s complete adjuvant (Sigma, U.S.A) (first injection) or incomplete adjuvant (Sigma) (other injections) (50 µg every 2 weeks). Three days after the last injection, spleen cells were fused with SP2/0 myeloma cells (NCBI 129, National Cell Bank of Iran, Pasteur Inst. of Iran, Tehran), using polyethyleneglycol (PEG 1500) (Sigma). Hybridomas were grown in DMEM culture medium (Sigma) containing 20% fetal calf serum (FCS) (Seromed, Germany), penicillin 100 IU/ml) and streptomycin (100 µg/ml) and supplemented with hypoxantine (1×10-4 M), aminopterin (4×10-7 M) and thymidine (1.6×10-5 M) (HAT) (Sigma).
Ten to fourteen days after fusion, secreting hybrids were identified by analysis of culture supernatants by the ELISA technique described below. Selected antibody producing cultures were cloned by limiting dilution process according to the conventional methods (17). Clones secreting antibody of desired reactivity were expanded in 25 and 75 cm2 flasks (Nunc, Denmark), harvested and cryopreserved in 40% FCS, 10% Dimethylsulfoxide (DMSO) (Sigma) and 50% RPMI-1640 culture medium (Sigma).
Detection of specificity of MAbs by indirect enzyme-linked immunosorbent assay (ELISA):Specificity of MAbs was determined by ELISA technique as described elsewhere (18). Briefly, microtiter polystyrene plates (Maxisorp, Nunc, Denmark) were coated with 1-10 µg/ml of purified myeloma IgG subclasses or polyclonal IgG in PBS (0.15 M, PH 7.2). Then 0.05 ml of culture supernatant was added. Appropriate dilution of HRP-conjugated sheep anti mouse Ig (prepared in our lab) was then added and the reaction revealed with O-phenylenediamine dihydrochloride (OPD) (Sigma) substrate. Finally, the reaction was stopped with 20% H2SO4 and the optical density (OD) measured by a multiscan ELISA reader (Organon Teknika, Boxtel, Belgium) at 492nm.
Isotype determination of MAbs by capture ELISA:
Goat antimouse IgG1, IgG2a, IgG2b, IgG3, IgA and IgM at 1/1000 dilution, were adsorbed on to the wells of a microtitre ELISA plate (Nunc). Isotypes of MAbs in culture supernatants were determined according to the ELISA technique mentioned above.
Affinity constant (Kaff) determination by ELISA:
We determined the Kaff by ELISA technique as described elsewhere (19). Briefly, ELISA plates (Nunc) precoated with f
Result :
Screening and selection of specific hybridomas:
Culture supernatants from growing hybridomas were screened by ELISA using a panel of four IgG myelomas with different subclasses, including their immunogens. Results obtained for these hybridomas with different specificity profiles are illustrated in Table 1.
Characterization of MAbs:Following cloning and subcloning, culture supernatant from the selected hybridomas was further characterized. Five MAbs belonged to IgG1, one MAb was IgG2a and the last one displayed IgG2b isotype (Table 2). Specificity of these MAbs was determined, using a panel of purified myeloma proteins, including IgG1(n=9), IgG2 (n=4), IgG3 (n=7) and IgG4 (n=6) subclasses.
According to ELISA and immunoblotting studies, these seven MAbs can be categorized into two groups: 1) Four IgG1, 2, 4 specific MAbs of which three (1F5A8, 8F9G7 and 6F11E1), react with conformational epitopes located on heavy chain of IgG1, 2, 4 (Figure 1) and an unusual IgG3 myeloma protein (Goe) bearing allotypic marker of the Mongloid populations [G3m(st)] displaying similar specificity to SPA. The fourth MAb (6F19C11) reacts with a linear epitope located on heavy chain of IgG1, 2, 4 subclasses. 2) Three IgG1, 2, 3 specific MAbs of which two (2F7G8 and 1F8G4) react with linear epitopes and the third one (7F14F7) reacts with conformational epitope located on heavy chain of IgG1, 2, 3 subclasses (Figure 2). Representative immunoblotting results are illustrated in Figures 3 and 4. All the MAbs reacted only with Fc, but not Fab fragments of their immunogens (Figure 5).
Cross-reactivity studies employing whole sera from a range of animal species indicated the most abundant cross-reactivity (71.4%) with monkey Igs, while no cross-reactivity was detected with hen and cat sera. While 4 MAbs displayed a restricted pattern of cross-reactivity, two MAbs [8F9G7 (IgG1, 2, 4 specific) and 7F14F7 (IgG1, 2, 3 specific)] displayed a broad pattern of cross-reactivity and one MAb [2F7G8 (IgG1, 2, 3-specific)], did not bind to any of the animal sera tested (Table 3).
Determination of affinity constant of MAbs: Ascitic fluids of three MAbs were available for purification by affinity chromatography using Streptococcal protein G column. The affinity constant (Kaff) of these MAbs was determined by ELISA. Triple serial concentrations of the antigens and MAbs were selected to construct the corresponding curves and to extrapolate the Kaff values using the formula given in Materials and Methods. The calculated average Kaff values are presented in Table 4.
Discussion :
Several anti-human IgG MAbs with specificity for Fc region-dependent epitopes common to three of four human IgG subclasses have been produced in the present study.
One group of our MAbs (n=4) was shown to be specific for isotypic epitopes common to IgG1, 2, 4, but not IgG3 subclasses. IgG3 subclass has different amino acid sequences at positions 276 and 291 in CH2 domain and positions 392, 422 and 435 in CH3 domain in comparison with other subclasses. In IgG1, 2 and 4 subclasses, these amino acids are Asn, Pro, Lys, Val and His, whereas in IgG3 subclass they include Lys, Leu, Asn, Ile and Arg, respectively (22). We suggest that these four MAbs recognize epitope (s) containing the differentiating amino acids located in CH2 and/ or CH3 domains common to IgG1, 2 and 4 subclasses, but not CH1 domain, because our results showed no reactivity of these MAbs with Fab fragments of IgG1 molecules (Figures 3 and 5).
MAbs with similar specificity have already been reported (HP6032, HP6033 (23); QF1, WC2, WC9 (24) and IG12 (25)). In contrast to HP6032 MAb, HP6033 showed also reactivity with IgG3 myeloma proteins having G3m (g) allotype and with one of two IgG3 myeloma proteins having G3m (b) allotype (23). These two MAbs were evaluated only by Immunoflurometric assay (IFMA) (23) and comparative binding study with SPA has not been conducted.
IG12 MAb reacts with a heat sensitive conformational epitope close to the hinge region of IgG and did not compete with Staphylococcus protein A (SPA) for IgG binding. Furthermore, the affinity constants of the previously produced anti IgG1, 2, 4 MAbs have not been calculated. Three of our anti IgG1, 2, 4 MAbs (1F5A8, 6F11E1 and 8F9G7), recognize conformational epitopes located to heavy chain of IgG1, 2, 4 but not IgG3 subclasses and a protein of IgG3 subclass (Goe protein) (Figure 1), a reactivity pattern similar to SPA (21).
The sequence of protein Goe resembled that of other ?3 molecules, except for the presence of tyrosine at position 296, alanine at position 339 and histidine and tyrosine at positions 435 and 436 (26). The only amino acid which is common between Goe protein and IgG1, 2 and 4 subclasses and is different between Goe protein and other IgG3 molecules, is histidine at position 435. Thus, it appears that histidine 435 plays an important role in binding to 1F5A8, 6F11E1 and 8F9G7 MAbs produced in present study. Reactivity of these monoclonal antibodies with polyclonal IgG subclasses was found to be similar to SPA.
It was previously shown that histidine 435 has a major role in binding to SPA (27). In the crystal structure of the complex between IgG1-Fc and domain B of SPA (28), it was revealed that His 435 is situated at the CH2-CH3 domain interface and is in contact with SPA. In IgG3 proteins from Caucasian populations the protein A contact residue is replaced by Arg. This lengthy side chain prevents the formation of favorable IgG-SPA contact, so that such IgG3 proteins do not bind protein A (29).
Since three of our MAbs (1F5A8, 6F11E1 and 8F9G7) displayed similar binding activity to SPA with regard to interaction with purified human myeloma proteins of IgG1, 2 and 4 and the IgG3 myeloma protein Goe, as well as human polyclonal IgG1, 2 and 4, but not IgG3 subclasses (Figure 1), the specificity of these MAbs resembled that of SPA. Our proposition is further supported by the fact that our MAbs only reacted with the unreduced form of IgG1, 2 and 4 subclasses and the Goe protein by immunoblotting (Figure 3). These results indicate that the epitopes recognized by these MAbs are conformational, depending on covalent interaction of the two heavy chains of IgG molecule.
The fourth MAb (6F19C11) in this category is completely different from the others as it does not recognize any protein of IgG3 subclass and it recognizes a linear epitope located to IgG1, 2, 4 subclasses (Figure 3).
The second group of our MAbs (three MAbs) was shown to be specific for isotypic epitopes common to
Acknowledgement :
We thank Dr. Mahmood Jeddi-Tehrani, Dr. Soheila Gharagozlou, Roya Ghods, Jalal Khoshnoodi and Azam Roohi for scientific consultations and preparation of myeloma proteins. This study was supported in part by a grant from the Research and Technology Undersecretary of the Ministry of Health, Treatment and Medical Education of Iran.
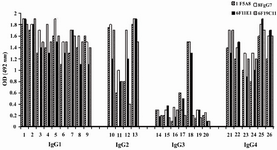
Figure 1. Reactivity of IgG1, 2, 4 specific MAbs with human IgG subclasses
Lanes 1-9, 10-13, 14-20 and 21-26 represent different IgG1, IgG2, IgG3 and IgG4 purified human myeloma proteins, respectively
|
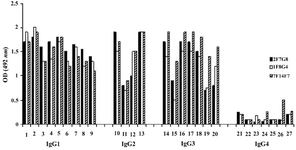
Figure 2. Reactivity of IgG1, 2, 3 specific MAbs with human IgG subclasses
Lanes 1-9, 10-13, 14-20 and 21-27 represent different IgG1, IgG2, IgG3 and IgG4 purified human myeloma proteins, respectively
|
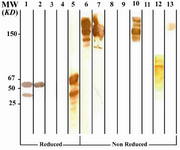
Figure 3. Immunoblot analysis of 6F19C11 MAb (anti-IgG1, 2, 4) reactivity with human IgG subclasses
Lanes 1 to 5 represent reduced forms of IgG1, IgG2, IgG3, IgG3 (Goe) and IgG4 paraproteins, respectively. Lanes 6 to 9 represent non reduced forms of the above mentioned proteins, respectively. Lanes 10 to 12 represent Whole molecule, Fab and Fc fragments of IgG4. Lane 13 represents polyclonal IgG3. *Fc fragments of IgG1 are contaminated with whole IgG molecule because of partial digestion
|
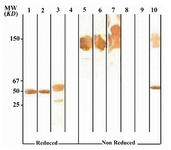
Figure 4. Immunoblot analysis of 2F7G8 MAb (anti-IgG1, 2, 3) reactivity with human IgG subclasses
Lanes 1 to 4 represent reduced forms of IgG1, IgG2, IgG3 and IgG4, respectively. Lanes 5 to 8 represent non reduced forms of the above mentioned proteins, respectively. Lanes 9 and 10 represents Fab and Fc fragments of IgG1, respectively
|
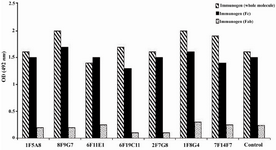
Figure 5. Reactivity of IgG1, 2, 4 and IgG1, 2, 3 specific MAbs with enzymatic fragments of human IgG
* Control represents a mouse MAb (8a4) with pan-IgG specificity
|
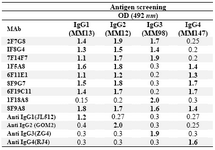
Table 1. Reactivity of selected MAbs with different human IgG subclasses
* Positive reactivity is shown in bold figures. 1F18A8 and 8F9A8 MAbs are IgG3 and Pan-IgG specific MAbs included as controls. Commercial IgG subclass monospecific MAbs, including JL512 (IgG1), GOM2 (IgG2), ZG4 (IgG3) and RJ4 (IgG4), are included as control MAbs for each IgG subclass
|
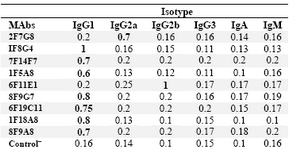
Table 2. Determination of the isotype of MAbs by ELISA
The results represent OD values obtained at 492 nm. Control‾ : Culture supernatant from SP2/0 myeloma cells
|
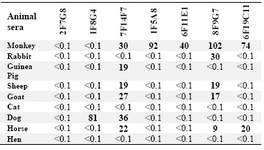
Table 3. Cross-reactivity of MAbs with animal sera
% Cross-reactivity is expressed relative to the value obtained for human pooled serum
|
![Table 4. Affinity constants of human IgG subclass-restricted MAbs determined by ELISA
*OD-50 represents the half maximum optical density obtained for a given concentration of h-IgG ([Ag]) and the corresponding MAb ([Ab]). The affinity constant (Kaff) for each selected concentration of Ag and Ab was determined using the formula described in the Materials and Methods](Images/Articles/28/tab4_small.png)
Table 4. Affinity constants of human IgG subclass-restricted MAbs determined by ELISA
*OD-50 represents the half maximum optical density obtained for a given concentration of h-IgG ([Ag]) and the corresponding MAb ([Ab]). The affinity constant (Kaff) for each selected concentration of Ag and Ab was determined using the formula described in the Materials and Methods
|
|