Flow Cytometric Analysis of 4-HPR-induced Apoptosis and Cell Cycle Arrest in Acute Myelocytic Leukemia Cell Line (NB-4)
-
Soleymani Fard, Shahrzad
-
Biology Department, Faculty of Basic Science, Science and Research Branch, Islamic Azad University , Tehran, Iran
-
Jeddi-Tehrani, Mahmood
-
Monoclonal Antibody Research Center, Avicenna Research Institute, ACECR , Tehran, Iran
-
Akhondi, Mohammad Mehdi
-
Reproductive Biotechnology Research Center, Avicenna Research Institute, ACECR , Tehran, Iran
-
Hashemi, Mehrdad
-
Molecular Genetic Department, Tehran Medical Branch, Islamic Azad University , Tehran, Iran
-
 M. Ardekani, Ali
Ph.D., Reproductive Biotechnology Research Center, Avicenna Research Institute, ACECR, Tehran, Iran, P.O. Box: 19615-1177, Tel: +98 21 22432020, Fax: +98 21 22432021, E-mail: Ardekani@avicenna.ac.ir
M. Ardekani, Ali
Ph.D., Reproductive Biotechnology Research Center, Avicenna Research Institute, ACECR, Tehran, Iran, P.O. Box: 19615-1177, Tel: +98 21 22432020, Fax: +98 21 22432021, E-mail: Ardekani@avicenna.ac.ir
-
Reproductive Biotechnology Research Center, Avicenna Research Institute, ACECR, , Tehran,, Iran
Abstract: In many acute leukemias, normal differentiation does not occur. However, in many cell lines derived from hematologic malignancies, differentiation or programmed cell death (apoptosis) can be induced by variety of agents including: Vitamin analogs, demethylating agents, cyclic AMP analogs and anti-proliferative agents. To the best of our knowledge there has been not any study specifically to analyze apoptotic and anti-proliferative effects of 4-HPR (a vitamin analog) in NB-4 cell line. To test whether this drug has activity in acute myeloid leukemia (AML), we first analyzed the anti-proliferative effect of 4-HPR in one AML cell line (NB-4) using MTT Assay. Next we tested whether this drug induced apoptotic cell death. The ability of this compound to induce apoptosis of cancer cells was examined by Annexin V-FITC Assay using Flow cytometry. We also analyzed the cell cycle progression by PI staining using flow cytometry. Using MTT assay, NB-4 cells exhibited increased inhibition of proliferation at micromolar concentrations of 4-HPR at 24, 48 and 72 hrs post treatment. Flow cytometry analysis indicates that 4-HPR is a potent inducer of in vitro apoptotic cell death, and cell cycle analysis revealed an increase in S phase population. In total, the results indicate that 4-HPR is a strong inhibitor of AML cell proliferation and a potent inducer of in vitro apoptotic cell death. Further studies are required to evaluate the in vitro effects of 4-HPR in AML blasts derived from AML patients.
Introduction :
Acute myelocytic leukemia (AML) is a malignant neoplasm of hematopoietic cells, often associated with abnormal proliferation of myeloid precursor cells, and cessation of cell differentiation at several stages of hema-topoietic precursors (1). AML is the most common type of leukemia often occurring in adults, with about 11,900 cases per year in the United States (1). The incidence of AML increases with age, and the peak of onset is in the sixth decade of the human life (1). AML contains less than 1% of all cancers, and about 34% of all types of leukemia (2).
Treatment phase of AML contains two general parts including remission induction and post-remission therapy. Cytarahine, An-thracycline, Daunoruhicin (DNR), and Thio-guanine are commonly used for remission induction (3). And some studies suggest that Idarubicin or Mitoxantrone (4), and the high-dose use of Cytarabine (5) are effective, too. Whether leukemia patients in the first remis-sion should receive bone marrow transplant immediately or after relapse has been a con-troversial issue (6). Although the successful results of transplant especially in persons less than 20 years of age are notable, the most significant effects of bone marrow transplant has been seen in patients failing chemo-therapy (6). Despite all progresses in the treat-ment of AML, patients with this cancer are faced with some complications and second malignancies (7).
Since most patients with AML will experi-ence the relapse, and long-term survival remains unresolved, there is a continued need to discover new strategies for treatment of leukemia. Recently, with the increase of laboratory proofs, researchers have begun targeted treatment of AML based on differ-entiation induction (8-10). Researchers have so far evaluated many differentiation inducing agents which affect the proliferation and differentiation of tumor cells or can induce apoptotic cell death in cancer cells (11-13).
One of the synthetic analogs of ATRA (all-trans-retinoic acid) is N-(4-Hydroxyphenyl)-all-trans retinamide (fenretinide) which is also known as 4-HPR (14). The substitution of the carboxyl group of ATRA with a 4-hydrox-yphenyl group connected to an amide can reduce dominantly the side effects including liver toxicity. Moreover, since fenretinide is not able to induce point mutations and chromosomal aberrations, it is not considered to be a genotoxic agent (15). Although natural retinoids such as ARTA and 13-cis-retinoic acid often induce differentiation in cancer cells or cause cessation of the cell cycle, fenretinide triggers some specific biological effects (16). Some of these effects are the generation of Reactive Oxygen Species(ROS) and lipid second messengers that seems to be involved in the induction of apoptosis in transformed and malignant cells in vitro (17, 18).
There is little information available about the functions of 4-HPR in myeloid malig-nancies. Therefore, in the present study, the effects of 4-HPR in acute myelocytic leuke-mia cell line (NB-4) were evaluated. Results have shown great effects of 4-HPR in inhibit-ing the growth and proliferation of AML cells (NB-4). Furthermore, 4-HPR was found to be a strong inducer of apoptosis in these cells.
Materials and Methods :
Cell lines:
The acute myeloid leukemia cell line
(NB-4) was purchased from Iran cell bank. Human NB-4 cell line was grown in RPMI 1640 culture medium (GIBCO, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS, GIBCO, Grand Island, NY).
MTT assay:
Cell proliferation was evaluated by the MTT [3, (4, 5-dimethylthiazol-2-yl)-5-(3-car-boxymethoxyphenyl–2 (4-sulfophenyl)-2H-tetrazolium] (SIGMA-ALDRICH®, stein-beim, Germany) assay. NB-4 cells were washed twice in RPMI 1640 by centrifugation at 300×g for 5 min. Cell concentration and viability were determined by hemocytometer counts of cells with 0.1% trypan blue dye.
5 × 104 cells were plated in a total volume of 100 µl in 96-well plates (FALCON, USA). Then, cells were suspended in medium with 4-HPR (SIGMA-ALDRICH®, Steinem, Germany) at concentrations of 1, 2.5, 5, 7.5, and 10 µM. The cells were incubated for 24-72 hrs at 37 °C in a humidified 5% CO2 atmosphere. Following the incubation, 0.01ml of MTT solution (at a final concentration 0.5 mg/ml) was added per well. The plates were then incubated for additional 3 hr at 37 °C in a humidified 5% CO2 atmosphere. After incubation, cells were deposited by centrifugation at 300 ×g, and the medium was removed. 100 µl of stop solution (isopropanol containing 0.04% HCl) was added per well. Immediately after solving the blue formazon crystals, the absorbance of samples was read using a 96-well plate reader (ELISA Reader, Antus 2020) at a wave length of 570 nM. Each data point was determined eight times prior to analysis. Statistical analysis of the cell proliferation assay was performed with the Microsoft Excel program version 2007.
Microscopy:
According to differences that exist between apoptotic and necrotic cells, two fluorescent dyes (AnnexinV-FITC and Propidium Iodide (SIGMA-ALDRICH®, Steinem, Germany)) were used in this study to analyze the apopto-tic and necrotic cells, respectively. NB-4 cells were treated by 4-HPR at concentration
7.5 µM. After 6 hr, cells were washed and stained by two fluorescent dyes. Then, they were analyzed by fluorescence microscope.
Apoptosis assay:
This procedure is based on evaluating apoptotic cells by detecting the phosphatidyl-serines. Annexin V-FITC is a fluorescent probe binding to phosphatidylserine.
In this study, 1 × 106 cells/ml suspension of NB-4 cells was induced for apoptosis by addition of several concentration of 4-HPR
(1, 2.5, 5, 7.5 µM). 1 x 106 cells/ml suspension of non-induced NB-4 cells was established as a negative control. Both control and test samples of NB-4 cell cultures were incubated for 24 hr in a 37 °C, 5% CO2 incubator. Following the incubation, the cells were washed twice with DPBS (Dulbecco's Phos-phate Buffered Saline). After that, the cells at concentration of about 1 × 106 cells/ml were resuspended in 1X binding buffer. 500? of the apoptotic cell suspension was added to a plastic 12 × 75 mm test tube, and 500? of the non-induced cell suspension was added to a second plastic tube.
Next, 5? of AnnexinV-FITC (SIGMA-ALDRICH®, Steinem, Germany) and 10? of propidium iodide (SIGMA-ALDRICH®, Steinem, Germany) were added to each cell suspension. Then the tubes were incubated at room temperature for exactly 10 min and protected from light. Finally, the fluorescence of the cells was immediately determined by a flow cytometer (Partec FloMax, version 2.3).
In order to adjust the flow cytometer for evaluating the apoptotic effects of anticancer drug in NB-4 cell line, a positive and a negative control sample was used. As a positive control, apoptosis was induced in a
1 × 106 cells/ml suspension of AML cells by addition of 1µg/ml Staurosporine (Figure 1A). Flow cytometric analysis was performed using Partec FloMax software.
Cell cycle analysis:
The DNA content during cell cycle steps were evaluated with flow cytometry. In brief, 5 × 106 cells were treated with 4-HPR at concentration 5 µM. After 24 hr, the cell pellets were washed and re
Result :
Anti-proliferative and inhibitory effects:
First, the anti-proliferative effects of 4-HPR were analyzed using the MTT assay. To assess cell proliferation, Microsoft Excel 2007 software was used to graph all data from MTT assay. Eight samples were tested for each drug concentration and each experiment was repeated three times prior to MTT analysis. Results of one representative experi-ment are shown in Figure 2. 4-HPR inhibits the growth of NB-4 cells in a concentration dependent manner. The effective dose that inhibits 50% growth (ED50) of these cells was calculated; 4-HPR inhibits the growth of NB-4 cells with an ED50 of approximately 5 µM after 24 hr treatment (Figure 2). The most significant inhibitory effects of 4-HPR in NB-4 cells were around 80% at 10 µM and 7.5 µM after 24 hr and 48 hr treatments, respectively.
Morphological study of the apoptotic and necrotic cells:
Figure 3 (Panel F) shows NB-4 cells stained with fluorescent dyes [AnnexinV-FITC (A) and Propidium Iodide (B)] after treatment with 4-HPR, respectively. As the pictures of inverted phase microscope show (Figure 3A, Panel P), apoptotic cells have a dense chromatin and still maintaining their cell membrane during apoptosis. However, in necrotic cells, the cell membrane is complete-ly destroyed and their cell content were thrown out (Figure 3B, Panel P).
Apoptosis:
To determine whether the growth inhibition by 4-HPR in NB-4 cells was associated with the induction of apoptotic cell death the apoptosis index was evaluated using the AnnexinV-FITC staining method (Figure 1). According to this method, NB-4 cells were evaluated by flow cytometry 24 hr post treatment with the anticancer drug. Stauro-sporine was used to induce apoptosis in NB-4 cells as a positive control. It is also known as a strong inducer of apoptosis in several cell lines, and it is a potent inhibitor of phospho-lipid/ Ca2+ dependent protein kinase (protein kinase C) (19). Many studies have shown the apoptotic effects of Staurosporine in several mammalian cell types (20). All data were analyzed by specific software Partec FloMax. Although the percentages which are shown in Figure 1 demonstrate the dose-dependent increase in the apoptotic fraction at first, however these percentages were significantly decreased at the highest concentration used in this assay (7.5 µM). The population of cells that underwent necrosis was increased with higher 4-HPR concentration. The most apo-ptotic fraction of NB-4 cells measured by flow cytometry was more than 60% at concentration 5 µM of 4-HPR.
Cell cycle analysis:
In this study, the DNA content of NB-4 cells during cell cycle was measured in order to obtain information about the cell cycle progression. Since it has been previously shown that 4-HPR causes the cessation of cell cycle in the G1 to S phase (21), the effect of 4-HPR on progression of NB-4 cell cycle was tested in this study.
Figure 4 shows the distribution of DNA content during cell cycle in NB-4 cells. In control samples (untreated NB-4 cells), 51.59% of all cells accumulated in G0/ G1 phase, 8.66% in S phase, and 39.74% in G2 M phase. Treatment of NB-4 cells with 4-HPR at concentration of 5 µM causes a decrease (about 11%) in the proportion of cells in G0/ G1 phase (Figure 4). Cells were observed to significantly accumulate in S phase (27.74% in treated cells versus 8.66% in control samples).
Discussion :
Over the recent years, it has been demon-strated that retinoids regulate biological pro-cesses including cell proliferation, differenti-ation, and apoptosis (22). Retinoids also modu-late cellular functions at many levels which are deregulated during carcinogenesis.
4-HPR is one of the synthetic derivatives of vitamin A (17). In many investigations, 4-HPR has shown cytotoxic activity against several cancer cell lines such as neuroblastoma, breast cancer, prostate cancer, colon cancer, ovarian cancer, bladder cancer, and human head and neck squamous carcinoma (23 - 25). Furthermore, it is proved that 4-HPR affects the hematopoietic neoplasms; for example, this drug inhibits the growth of ATRA-resistant myeloid leukemia cell lines, T lymphoma cells, and T lymphoblastic leuke-mia cells (26, 27). 13-cis-Retinoic acid increases the event-free survival of children with high-risk neuroblastoma, and ATRA (all-trans-retinoic acid) has recently become an important part of Acute Promyelocytic Leuke-mia (APL) treatment (28).
In animal models, it is proved that fen-retinide (4-HPR) is an effective agent to prevent adverse side effects of chemotherapy against several cancers including breast cancer (29), prostate and pancreas cancer (30), and skin tumors (31, 32). Moreover, clinical trials have shown that fenretinide reduce the progress rate of prostate cancer in men who are diagnosed in early stages of disease (33). This drug can also prevent the development of ovarian cancer and second breast malig-nancies in women who are being treated against breast cancer in early stages (34). How-ever, very few is known about the effects of 4-HPR in acute myelocytic leukemia. There-fore, the mechanisms by which 4-HPR acts in these cell systems are not totally understood.
This study evaluated the effects of 4-HPR in acute myelocytic leukemia cell line (NB-4) which have not been reported previously. We have demonstrated that 4-HPR strongly inhibits the growth of NB-4 cells. As we have shown in Figure 2, inhibition of proliferation of NB-4 cells was about 80% at 4-HPR concentration of 7.5 and 10 µM. In this study, the inhibitory effects of 4-HPR in NB-4 cells after 72 hr treatment was shown to be less than the effects after 24 hr and 48 hr treat-ment. This effect was not an artifact, since the anti-proliferative effect of 4-HPR in NB-4 cells was evaluated three times and the results were approximately the same each time. This effect might have happened due to reduced presence of 4-HPR in the test culture medium. Such reduction may have allowed the viable cells to proliferate and grow in culture medium.
We were interested to test the ability of 4-HPR to induce apoptosis in this cell line; therefore flow cytometry was employed. The flow cytometric results show that 4-HPR is a potent inducer of programmed cell death (apoptosis) in NB-4 cells (Figure 1). These data show that 4-HPR at 5 µM could induce the maximum amount of apoptosis (60.34%) in these cells. However, flow cytometric analysis indicated a continuous increase in the fraction of necrotic cells with increasing concentrations of 4-HPR. At concentration of 7.5 µM, the percentage of apoptotic cells decreased, and more than 40% of cells died through necrosis. This effect may be due to cytotoxic effects of 4-HPR in concentrations more than 5 µM.
Initially we demonstrated that 4-HPR is a potent inhibitor of cell proliferation and a strong inducer of apoptosis in NB-4 cells. Next, we evaluated the effects of 4-HPR on the progression of NB-4 cell cycle. This can give useful information about the specific action of cytotoxic drugs. A variety of methods have been used for measuring the DNA content of cells. All methods use dyes which bind specifically to nucleic acids, and upon binding their fluorescence is enhanced.
As previous studies have shown, one of the essential steps to initiate terminal differenti-ation is cell cycle arrest (35). Even though G1 arrest has been known as a central step i
Acknowledgement :
This work was supported by a grant from Monoclonal Antibody Research Center at Avicenna Research Institute. We thank Ms. Leila Eini for her assistance in cell culture and Mr. Torkabadi and Mrs. Vahedian for their help in flow cytometry analyses. We specially thank Dr. Jeddi Tehrani for his support and help throughout this study.
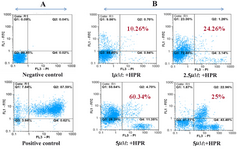
Figure 1. (A) Negative and positive control, (B) Apoptotic effect of 4-HPR in NB-4 cells at concentration 1, 2.5, 5 and 7.5M. Lower left quadrants, viable cells. Lower right quadrants, necrotic cells. Upper left quadrants, early apoptotic cells. Upper right quadrants, nonviable late apoptotic cells.
|
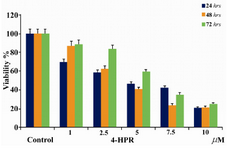
Figure 2. Anti-proliferation effects of 4-HPR in NB-4 cells after 24, 48, and 72 hrs. Eight samples were tested for each drug concentration and each experiment was repeated three times prior to MTT analysis
|
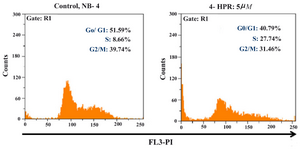
Figure 3. Morphological study of the apoptotic (A) and necrotic (B) cells using inverted phase microscope (Panel P) and fluorescence microscope (Panel F)
|
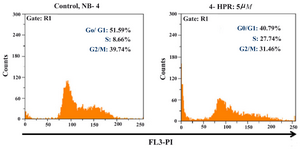
Figure 4. Effects of 4-HPR in NB-4 cell cycle progression after 24 hr, stain with PI
|
|