Paroxetine Can Enhance Neurogenesis During Neurogenic Differentiation of Human Adipose-derived Stem Cells
-
Jahromi, Maliheh
-
Department of Anatomical Sciences, Faculty of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran
-
 Razavi, Shahnaz
Department of Anatomical Sciences and Molecular Biology, Faculty of Medicine, Tehran University of Medical Sciences, Tel: +98 311 7922455, E-mail: razavi@med.mui.ac.ir
Razavi, Shahnaz
Department of Anatomical Sciences and Molecular Biology, Faculty of Medicine, Tehran University of Medical Sciences, Tel: +98 311 7922455, E-mail: razavi@med.mui.ac.ir
-
Department of Anatomical Sciences, Faculty of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran
-
Amirpour, Nushin
-
Department of Anatomical Sciences, Faculty of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran
-
Khosravizadeh, Zahra
-
Department of Anatomical Sciences and Molecular Biology, Faculty of Medicine, Tehran University of Medical Sciences, Tehran, Iran
Abstract: Background: Some antidepressant drugs can promote neuronal cell proliferation in vitro as well as hippocampal neurogenesis in human and animal models. Furthermore, adipose tissue is an available source of adult stem cells with the ability to differentiate in to multiple lineages. Therefore, human Adipose-Derived Stem Cells (hADSCs) may be a suitable source for regenerative medical applications. Since there is no evidence for the effect of Paroxetine as the most commonly prescribed antidepressant drug for neurogenic potential of hADSCs, an attempt was made to determine the effect of Paroxetine on proliferation and neural differentiation of hADSCs.
Methods: ADSCs were isolated from human abdominal fat. These cells differentiated to neuron-like cells and were treated with Paroxetine. 3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyl tetrazolium bromide (MTT) assay and immunofluorescence technique were used for assessment of cell proliferation and neurogenic differentiation potential of induced cells, respectively.
Results: MTT assay analysis showed that Paroxetine significantly increased the proliferation rate of induced hADSCs (p<0.05), while immunofluorescent staining indicated that Paroxetine treatment during neurogenic differentiation could enhance the mean percentage of Nestin and MAP2 (Microtubule-associated protein-2) positive cells but the mean percentage of GFAP (Glial acidic fibrillary protein) positive cells significantly decreased relative to control group (p<0.05).
Conclusion: Our results provide evidence that Paroxetine can promote proliferation and differentiation rate during neurogenic differentiation of hADSCs. Moreover, Paroxetine can reduce gliogenesis of induced hADSCs during neurogenic differentiation.
Introduction :
Depression is a severe and prevalent mental disease, characterized by a constant low mood and loss of all interest 1; for example, more than 16% of US adults are suffering from Major Depression Disorder (MDD) as the most common disease 2. Treatment with antidepressant drugs can be efficacious in the treatment of mild to severe depression 1.
Previous studies demonstrated that antidepressants increase neuronal cell proliferation in vitro 3-8 and enhance hippocampal neurogenesis in human and animal models 5-7. Paroxetine is functionally classified as a Selective Serotonin Reuptake Inhibitor (SSRI) 9-12, which is used in the treatment of depression 13, generalized anxiety disorder 14, obsessive-compulsive disorder 15, panic 16 and post-traumatic stress disorder 17.
This drug potently and selectively inhibits synaptosomal reuptake of serotonin by binding to the 5-HT (5-hydroxytryptamine) transporter in the neuronal membrane and blocking reuptake of 5-HT from the synapse into the presynaptic nerve terminal. This inhibition should result in accumulation of serotonin 18, some dopamine reuptake inhibitors, monoamines and other neurotransmitter amines in the synaptic cleft and therefore facilitation of serotoninergic transmission in the central nervous system 19.
Previous studies have revealed that enhanced serotonin concentration in synaptic cleft may contribute to stimulation of morphogenesis in the hippocampus and cause to maintain mental balance 20,21. There is some evidence that dysfunction in serotonergic activity may be involved in the pathophysiology of depression 22. However, increase of neurotransmitter amines in extracellular levels takes place in several weeks or months 19,23-25. It is well established that depletion of serotonin in the brain results in suppression of neurogenesis in the adult hippocampal, whereas elevation of the level of monoamines, serotonin and noradrenalin increases the rate of neurogenesis 26,27. However, the majority of the antidepressant drugs elevate the level of serotonin, and therefore, one of their effects could be the increase of the neurogenesis rate in hippocampus 26. Some hypotheses link depression to the decline of neurogenesis in hippocampal and suggest that the increase in the rate of neurogenesis plays an important role in the mood-stabilization effect of the antidepressant drugs 26,28.
Mesenchymal Stem Cells (MSCs) are prototypical adult stem cells with the capacity for self-renewal and differentiation with a broad tissue distribution 29, that can be isolated from many adult tissues, such as bone marrow and most connective tissues of the body 30,31. MSCs can differentiate into cells of the mesodermal, endodermal and ectodermal lineages, such as adipocytes, osteocytes and chondrocytes, fat, cartilage and cardiomyocytes, endothelial cells, lung epithelial cells, hepatocytes, neurons, and pancreatic islets 29,32-34.
MSCs can interact with cells of both the innate and adaptive immune systems, also secrete a variety of factors that induce tissue repair, stimulate proliferation and differentiation of endogenous tissue progenitors 35,36. Therefore, MSCs are attractive candidates for clinical applications in autologous transplantation 37,38, and also are effective in the treatment of various degenerative diseases and tissue damage 29,30.
Neural tissue has historically been regarded as having poor regenerative capacity but recent advances in the growing fields of tissue engineering and regenerative medicine have opened new hopes for the treatment of nerve injuries and neurodegenerative disorders. Adipose tissue has been shown to contain a large quantity of Adult Stem Cells (ASCs). These cells can be easily harvested with low associated morbidity. These cells after neural induction express neural markers and show neuron-like morphology 39-42. It has been suggested that neural like cells can be used for a wide variety of therapeutic applications 43,44.
On the basis of these findings, it was interesting to determine the influence of antidepressant drug, Paroxetine, on the proliferation and differentiation of human adipose-derived stem cells into neural lineage.
Materials and Methods :
Tissue collection and isolation of human adipose-derived stem cells (hADSCs): Samples of adipose tissue were obtained from abdominal fat of three patients (age range: 20-45 years) during abdominoplasty surgery, after receiving informed consent. Human ADSCs were isolated as previously described 43.
Briefly, adipose tissues were washed several time with sterile phosphate-buffered saline (PBS) to remove contaminating debris and red blood cells. Then adipose tissue was enzymatically dissociated with 0.075% collagenase I in PBS for 30 min at 37°C. The collagenase was neutralized with an equal volume of Dulbecco's Modified Eagle Medium (DMEM): F12/10% Fetal Bovine Serum (FBS) (Gibco, BRL, Paisley, UK) and centrifuged for 10 min at 1200×g. The cellular pellet was resuspended in growth medium (DMEM: F12/10% FBS and 1% penicillin/streptomycin solution). Cell cultures were maintained in T25 flasks for 4-5 days in a 37oC incubator with 5% CO2 until they become confluent. Then, hADSCs were passaged at the ratio of 1:3.
Cell cultures were assessed using bright field and phase contrast microscopy (Nikon Eclipse TS100). The changes in morphology of isolated stem cell and neurogenic induced cells were analyzed following the Paroxetine exposure for 2, 4 and 6 days. All chemicals, except where specified otherwise, were purchased from Sigma-Aldrich (St. Louis, MO).
Neurogenic differentiation of human ADSCs: For neural induction, hADSCs passage 3-6 was used. HADSCs were plated in low attachment plastic tissue culture dishes in culture medium that contained: DMEM: F12, 2% B27, supplemented with 20 ng/ml basic Fibroblast Growth Factor (bFGF) and 20 ng/ml Epidermal Growth Factor (EGF). The media were renewed every 2 days up to 6-7 days.
After replating the neurospheres dissociated cells on cover slips in a 24-well plate at a density of 2×104 /cm2, they were incubated in neurobasal medium(NB) supplemented with 5% FBS, 1% L-glutamine, 1% none essential amino acids, 1% N2 supplement and 2% B27 for 1 week (the growth factors and supplements are all from Gibco, BRL, Paisley, UK). Depending on the purpose of the experiments, the cells were cultured in neural induction medium with or without 1 μM Paroxetine in treated and control groups, respectively for 6 days.
3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyl tetrazolium bromide (MTT) assay: For assessment of proliferation rate in Paroxetine and control groups, MTT assay was performed 2, 4 and 6 days after Paroxetine treatment. Differentiated cells with PBS were washed; the MTT assay was performed as previously described 32. Briefly, 2×103 cells/well were seeded on 96-well plates and grown in the presence of Paroxetine at 1 μM or absence of Paroxetine. 100 μl of DMEM and 10 μl of a MTT solution (0.5 mg/ml) were added to each well and incubated for 4 hr. The MTT solution was removed from cell cultures and 100 µl of DMSO (Dimethyl Sulfoxide) was added to extract the MTT formazan. The absorbance of each well was measured by microplate reader (Hiperion MPR 4+, Germany) at 540 nm.
Immunocytochemistry staining: The induced cells were fixed in 4% paraformaldehyde for 20 min at room temperature and after being rinsed twice with PBS, the cells were permeablized using PBS containing 2% Triton X-100 at room temperature for 30 min. Primary antibodies diluted in blocking solution consistent 10% goat serum and 1 mg/ml Bovine Serum Albumin (BSA/PBS) for 2 hr at 37oC. Then, the cells were incubated with primary antibodies overnight at 4oC. After washing with PBS, the cells were incubated with FITC conjugated secondary antibodies for 2 hr at 37oC. The differentiated cells were reacted with antibodies against mouse anti-Nestin (1:300, Abcam, Cambridge, MA, USA), mouse anti-microtubule associated protein2 (MAP2, 1:300, Abcam, Cambridge, MA, USA), mouse anti-glial fibrillary acidic protein (GFAP, 1:600, Abcam, Cambridge, MA, USA) and anti-mouse FITC-conjugated IgG antibody (1:500, Abcam, Cambridge, MA, USA). For quantitative analysis, the cells were incubated with 4, 6-diamidino-2-phenylindole (DAPI, 1:1000) for 2 min at room temperature.
Preparations were examined using a fluorescence microscope (Olympus BX51, Japan). The numbers of positive cells to each slid were counted as percentages of the total DAPI-stained cell population. For quantitative assessment of cell differentiation in control and Paroxetine treated cells, the relative numbers of cells expressing different markers like mature neurons (MAP2), astrocytes (GFAP), and precursor neuronal cells (Nestin) were counted as percentages of the total DAPI-stained cell population. ImageJ software was used for merging the pictures.
Statistical analysis: Cell proliferation and neural differentiation data were analyzed using one-way ANOVA (SPSS (PASW) 17Inc, Chicago, IL). All the data were shown as means± Standard Error of Mean (SEM). Experiments with two groups were subjected to a one-way ANOVA and p<0.05 was taken as significant and p<0.001 was taken as highly significant to indicate levels of statistical significance.
Results :
Morphologic changes of hADSCs following neurogenic differentiation: In the present study, the isolated stem cells were characterized early during primary culture by a slightly heterogeneous morphology. It was noted that hADSCs were stretched with spindle-like shape fibroblastic morphology after two or three passages (Figure 1A).
Few cells adhered to the surface of flasks. A lot of small spheres of floating cells appeared after 7 days in pre induction medium (neurospheres), more than 90% of hADSCs converted into neurospheres (Figure 1B).
Six days after treatment with Paroxetine, a lot of singled neurosphere cells would gain contracted cell bodies, and the cell processes would grow much longer in control group (Figure 1C) and some bipolar, spindle-like cells began to appear 10 days after treatment and almost all cells were bi- or multi-polar (Figure 1D).
Proliferation rate of induced hADSCs: As shown in figure 2, as compared with control group, exposure to 1 μM Paroxetine did not have a significant effect on cell proliferation rate after 2 days, while, 1 μM Paroxetine treatment significantly increased the cell proliferation relative to control group after 4 and 6 days post induction (p≤0.01).
Immunocytochemical analysis of induced hADSCs: Neural differentiation was analyzed through the detection of the mean percentage of positive cells of neural markers (Nestin and MAP2), and GFAP as a marker of astrocyte by immunofluorescence technique (Figure 3). The results in figure 4 indicated that after treatment with 1 μM Paroxetine, there was no significant difference between induced cells and control groups for percentage of MAP2 positive cells.
Furthermore, after treatment with Paroxetine, the mean percentage of GFAP positive cells significantly decreased in Paroxetine treated group (p<0.01), relative to the control group, while analysis indicated that the mean percentage of Nestin positive cells significantly increased in the treated group in comparison to control group (p<0.01).
Quantification of immunostaining indicated that 26±7/1% of treated cells were positive for Nestin, which was significantly different from control group (10±3.7%). Immunostaining analysis showed that the mean percentage of GFAP positive cells was 7±1.1% in Paroxetine treated group as compared to control group (32±18%), which demonstrated that the mean percentage of GFAP positive cells significantly decreased in the treated group. Finally, the mean percentage of MAP2 positive cells was 62±10% that increased in Paroxetine treated group in comparison with control group (38±31) (Figure 4).
Discussion :
New neurons continue to be born in the adult mammal's brain. This type of adult neurogenesis is mainly restricted to the hippocampus and the olfactory bulb 45. These cells may contribute to tissue repair in some neurological diseases and screen the candidate agents for neurogenesis in neurodegenerative diseases 46.
SSRIA antidepressant, Paroxetine, increases differentiation and promotes neuronal proliferation of hippocampal progenitor cells both in vivo and in vitro 47-49. Another study showed that antidepressant drug increased cell proliferation and enhanced differentiation in neural precursors derived from human Embryonic Stem Cells (hESCs) 50. In addition, there is strong evidence that in animals, stress reduces the atrophic properties and neurogenesis of neurons in the adult hippocampus 51,52. In contrast, long-lasting antidepressant treatment prevents and/or reverses these effects, both in humans and animal models 53-55.
Stem cells in hippocampus are few but adipose tissue represents an abundant, accessible and multipotent source of adult stem cells with treatment potential for neurodegenerative disorders via stem cell transplantation. Therefore, these cells can be appropriate candidates for treatment of neurodegenerative disorder.
Lau et al indicated that cell proliferation in the hippocampus may be involved in reproductive potential of male rodents, and Paroxetine treatment may affect sexual functioning through increased neurogenesis 56.
The direct effect of Paroxetine on the neurogenesis of Neural Stem Cells (NSCs) indicated that Paroxetine can promote the proliferation and differentiation of NSCs into neurons other than glial cells 48.
In the present study, for assessment of the effect of Paroxetine on proliferation rate and potential of neurogenic differentiation of ADSCs, 5 μM of Paroxetine was used according to Peng et al, but in 5 μM Paroxetine treatment, all of treated cells were dead. Then, lower doses of 2 μM concentration were used, but again all of treated cells were dead. Finally, in 1 μM of concentration of Paroxetine, no toxic effect on treated cell cultures was found.
Also, our previous study showed that sertraline as an antidepressant can enhance proliferation rate during neurogenic differentiation of hADSCs 57. Our result is consistent with findings of Peng et al which demonstrated that Paroxetine can increase proliferation and differentiation of hippocampus-derived neural stem cell as compared to control group. However, they showed that high concentrations of Paroxetine (20 μM and 50 μM) inhibit the proliferation of NSCs.
Verdi et al in 2014 described that citalopram can increase the neuronal-like cell differentiation and proliferation of bone marrow mesenchymal stem cells 58.
Recent studies showed that other antidepressants, such as, imipramine, venlafaxine (1 μM) 4, fluoxetine (1 μM) 4,25,59 and sertraline (1-5 μM) 60 increased the proliferation rate of NSCs with different sources.
Previous studies demonstrated that above antidepressant drugs can induce positive effect on proliferation and neurogenic differentiation 4,25,48,59,60, although there are dissimilarities in cell lines, duration of treatment, concentration and type of antidepressant drugs.
Previous studies demonstrated that increase of the expression of Brain Drive Neurotrophic Factors (BDNF) at mild concentration by Paroxetine could play a role for the proliferation and differentiation into neurons 47-49.
Conclusion :
In conclusion, our results suggest that Paroxetine can induce proliferation and differentiation of hADSCs during neurogenic differentiation. Moreover, the molecular mechanism of Paroxetine on hADSCs proliferation and differentiation is not well understood. Therefore, it is essential that the researches evaluate the effective factors and mechanisms which are involved in Paroxetine function.
Acknowledgement :
This study was supported by Isfahan University of Medical Sciences.
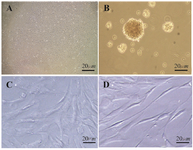
Figure 1. Phase contrast microscopic morphological changes of hADSCs following differentiation with 1 μM Paroxetine in vitro. A) Undifferentiated ADSC. B) After 6-7 days of culture in pre induction medium, the cells exhibited sphere shape; C) In control group, the induced cells with 1 μM Paroxetine formed contracted cell bodies with long cytoplasmic processes 10 days post induction, (D) In the treated group, the cell bodies became bipolar and multipolar 10 days post induction. Scale bar=20 µm
|
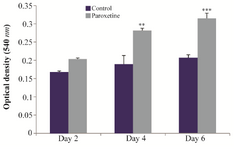
Figure 2. Determination of the mean of Optical Density (OD) for treatment with 1 μM Paroxetine in assessing the proliferation rate at 2, 4 and 6 days after induction. Compared with control group, exposure to 1 μM Paroxetine resulted in a significant increase of cell proliferation after 4 and 6 days (**p≤0.01,***p≤0.001).
|
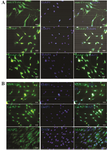
Figure 3. Immunocytochemical staining for specific neural markers in neurogenic differentiated cells (Nestin and MAP2), and GFAP as a marker of astrocyte treated with 1 μM Paroxetine, A) and control group B). In each experiment, the nuclei were counterstained with DAPI. Scale bar=100 µm.
|
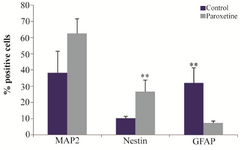
Figure 4. The mean percentage of immunoreaction of positive cells for Nestin, MAP2 and GFAP in treatment with 1 μM Paroxetine com-pared with control group. The mean percentage of MAP2-positive cells increased in the Paroxetine treated group as compared to control group, while the mean percentage of Nestin-positive cells significantly increased in the Paroxetine treated group as compared to control group (**p≤0.01), but the mean percentage of GFAP positive cells in the treated group significantly decreased relative to control group (**p≤0.01).
|
|