Retinoic Acid as the Stimulating Factor for Differentiation of Wharton’s Jelly- Mesenchymal Stem Cells into Hepatocyte-like Cells
-
Mortezaee, Keywan
-
Department of Anatomy, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran
-
Minaii, Bagher
-
Department of Anatomy, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran
-
Sabbaghziarani, Fatemeh
-
Department of Anatomy, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran
-
Ragerdi Kashani, Iraj
-
Department of Anatomy, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran
-
Hassanzadeh, Gholamreza
-
Department of Anatomy, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran
-
Pasbakhsh, Parichehr
-
Department of Anatomy, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran
-
 Barbarestani, Mohammad
Department of Anatomy, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran, Tel: +98 21 64432348, E-mail: barbarestani@sina.tums.ac.ir
Barbarestani, Mohammad
Department of Anatomy, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran, Tel: +98 21 64432348, E-mail: barbarestani@sina.tums.ac.ir
-
Department of Anatomy, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran
Abstract: Background: Wharton’s Jelly-Mesenchymal Stem Cells (WJ-MSCs) are pluripotent cells with differentiation capability into most cell lineages. The aim of the current work was to examine the role of Retinoic Acid (RA) in differentiation process of these cells into hepatocyte-like cells and determine the morphological and functional patterns.
Methods: Human WJ-MSCs were extracted, cultured and expanded; after approximately 95% of confluence, the cells were treated with hepatogenic media containing RA. The cells were subsequently analyzed for morphological changes, glycogen storage, albumin production, and specific gene expression.
Results: WJ-MSCs expressed high levels of CD90 (93.6%) and CD105 (90.7%), but low levels of CD34 (0.3%) and CD45 (0.8%). Albumin production had significant difference in the two groups (p≤0.05). The data showed specific characteristics in favor of considering the differentiated cells as hepatocyte-like cells such as obtaining morphologic, functional, and αFP and HNF1-α expression patterns which in turn were higher in cells exposed to RA.
Conclusion: Based on the data of present study, RA is an effective molecule in inducing differentiation of WJ-MSCs into hepatocyte-like cells; therefore, it may be considered as a promising factor for targeting therapy of liver disorders.
Introduction :
Liver problems are one of the most dangerous diseases with high rate of mortality in the world 1. Although liver transplantation is a good alternative treatment for chronic liver diseases, limited numbers of donor livers attract scientists about the possibility of using cell replacement therapy especially by using stem cells 2. Preclinical findings from animal experiments have shown the considerable potential of Mesenchymal Stem Cells (MSCs) in a wide variety of diseases 3. MSCs are plastic adherent cells with a specific surface phenotype that have the capacity to self-renew and differentiate into various lineages including bone 4, cartilage 5, adipose 6, endothelial 7, muscle 8, and nerve 9 cells with specific culture conditions. Different sources of MSCs include adipose tissue, muscle, skin, Bone Morrow (BM), articular synovial and Wharton’s Jelly (WJ) 10.
The yield of Bone Marrow-derived Mesenchymal Stem Cells (BMMSCs) is substantially low and diminishes progressively with advancing donor age; therefore, many researchers were looking for an alternative source, especially Wharton’s Jelly-Mesenchymal Stem Cells (WJ-MSCs) 11. WJ-MSCs like BMMSCs express type I MHC molecule, MSC markers (SH2 and SH3), and also adhesion molecules (CD44 and CD105), but not type Π MHC and hematopoietic markers (CD34 and CD45) 12-14. WJ-MSCs are primitive, uncontaminated, immunotolerable 12 and are a low cost source for stem cells that can be easily obtained and propagated in culture without any invasive medical procedures and ethical issues. These cells could be induced to form other cell lineages such as neuron, glial 15, germ 16, endothelial 17 and insulin producing 18 cells.
FGF4 is the main factor for endodermal patterning during embryogenesis 19, and HGF is the principal factor for hepatocyte differentiation 20, proliferation, and regeneration and development 21. Retinoic Acid (RA) is considered as the most common differentiating factor in clinical trial 22. Scientists have shown differentiation of hematopoietic and non-hematopoietic stem cells into hepatocyte-like cells 23,24. A recent study attested to the beneficial effects of RA in differentiation of hepatic progenitor cells into hepatocyte-like cells 25. Differentiation potential of human WJ-MSCs is the most interesting field among scientists as a promising cell source for regenerative medicine 26. Based on the fact that no survey has been conducted so far on using RA as an inducer for hepatocyte differentiation potential of WJ-MSCs, the present study was designed for answering this question.
Materials and Methods :
Isolation and cultivation of WJ-MSCs: By considering all ethical aspects, fresh umbilical cords were aseptically collected from full term infants after cesarean section by taking into account the patient's permission, the policy of Mirza Kochak Khan Hospital, and Ethical Committee of Tehran University of Medical Sciences, Tehran, Iran. Samples were stored in a solution containing 1 ml amphotericin B (Sigma-Aldrich, USA), 20 ml Dulbecco’s modified eagles medium (DMEM, Sigma-Aldrich, USA), and 1 cc heparin in a standard way for processing, during the 2-4 hr time period from obtaining. MSCs were isolated through explant method. In the first step, specimens were rinsed several times with phosphate buffered saline (PBS, Sigma-Aldrich, USA) for removing vessels' blood; thereafter, they were dissected into 0.5 cm2 pieces for further WJ removal. Following that, they were cultured in a DMEM supplemented with 15% fetal bovine serum (FBS, Sigma-Aldrich, USA), amphotericin B (1 µg/ml), penicillin (100 U/ml) and streptomycin (100 mg/ml) and incubated at 37°C with saturated humidity and 5% CO2 for 7 days. Eventually, WJ pieces were taken away and medium changes were carried out twice a week. Upon achieving roughly 90% confluence, the cells were trypsinized (0.25% trypsin-EDTA, Gibco, USA) and passaged. For further assays, passage 3 cells were used.
Flow cytometry: For flow cytometry analysis, 1×105 cells were used. Cells were incubated for 20 min in a dark condition and room temperature with an appropriate amount of fluorescence conjugated monoclonal antibodies for MSC surface positive (CD105 and CD90) and negative (CD34 and CD45) markers (all from Ebioscience, USA). Subsequently, the cells were washed and centrifuged for 5 min at 800 g, then read on a FACS Calibur flow cytometer (Beckman, Fullerton, CA, USA).
Hepatocyte differentiation of WJ-MSCs: Cells at the density of 1.5×104/cm2 were serum deprived for 2 days, then exposed into hepatogenic media cantaining 5 cc low glucose DMEM supplemented with 1 µmol/ml dexamethasone, 20 ng/ml HGF, 20 ng/ml FGF4, 10-4M ascorbic acid 2-phosphate, 100 mg/ml streptomycin, 1 mM sodium butyrate, and 1 µM RA (all from Sigma-Aldrich, USA) for a duration of 28 days; cell media were replaced twice weekly. Evaluations for morphologic and functional characteristics were done on day 28 and for specific hepatocyte markers on days 14 and 28, owing to different expression patterns of the chosen markers.
Reverse transcriptase polymerase chain reaction (RT- PCR): The hepatocyte’s alpha-fetoprotein (αFP) and hepatocyte nuclear factor-1α (HNF-1α) genes expression levels were tested by RT- PCR technique. Total RNA extraction and complementary DNA (cDNA) synthesis (both from Invitrogen, USA) have been done according to the manufacturer's protocol; after an initial denaturation at 94°C for 5 min, cDNA was subjected to 33 cycles of PCR. To normalize samples, the expression of β-actin as housekeeping gene was examined. Finally, products were observed on 2% agarose gel. The primers were shown in table 1.
Periodic acid-schiff (PAS) staining for glycogen detection: Functional feature of hepatocyte-like cells was assessed by PAS staining for glycogen storage. Initially, cells were fixed in 4% paraformaldehyde overnight; then, they were exposed to periodic acid and Schiff’s reagent (15 min for each step) and Mayer’s Hematoxylin (1 min). Further evaluation has been done under light microscopy (Olympus, Japan).
Albumin measurement: Human albumin concentrations in cell culture supernatant were evaluated using human albumin ELISA kit (Abcam, USA) based on the manufacturer’s protocol. Samples were harvested at weeks 1, 2, 3 and 4 of initial cell culture. Cultures were briefly washed with Wash Buffer and analyzed.
Statistical analysis: For comparing the mean differences for the groups and measuring changes in time periods, independent samples T-Test was used through SPSS 16 software (Microsoft, IL, USA). The p<0.05 was considered statistically significant.
Results :
Isolation and cultivation of WJ-MSCs: MSC detachment from WJ was carried out via explant method. The number of cells isolated from each 1 cm length of cord was 10-12×103. At first, the primary segregated cells had heterogeneous flattened fibroblast-like characteristics in terms of shape and processes (Figure 1A). After a few weeks of expansion in medium (Figures 1B and 1C), they obtained homogeneous features with the following colony formation (Figure 1D).
Flow cytometry: Human WJ-MSCs at passage 3 were characterized by flow cytometry analysis (Figure 2) for the evaluation of stem cell markers expression. The cells expressed considerable levels of CD90 (93.6%) and CD105 (90.7%), but slight levels of CD34 (0.3%) and CD45 (0.8%). The expression rate for CD90 was the highest.
Hepatocyte-like cells morphological characteristics: The cessation of cell proliferation was observed during serum free time period (Figure 3A). In the presence of RA, cells acquired shortened appendages and broadened and flattened shape (Figure 3B), whereas the changes were not remarkable in cells without exposure to RA after two weeks (Figure 3C). Three weeks after induction, RA led cells into developing polyhedral shapes (Figure 3D) which were in contrast with the shapes of cells in the absence of this factor (Figure 3E). Abundant cytoplasmic granules appeared markedly in exposed cells after four weeks (Figure 3F vs. 3G). Hepatocyte-like cells could obtain hexagonal structure with large nucleus (Figure 3H) and further canalicular architecture (Figure 3I) following prolonged culture in exposed cells.
RT-PCR analysis of hepatocyte-specific genes: Expression patterns of αFP and HNF1-α were analyzed in hepatocyte-like cells using RT-PCR technique. The rate of αFP expression, as the early marker, in cells exposed to RA was the highest on day 14 with a downside trend over time. On the other hand, the rate for HNF1-α, as the secondary marker, was highest in RA receiving cells on day 28; however, its expression was less during the first two weeks with or without RA (Figure 4).
Functional characterization of hepatocyte-like cells: Glycogen storage was appraised in hepatocyte-like cells by PAS staining method. The depository was noticeable in RA exposed cells which was in contrast with the ones not exposed at week four (Figures 5A and 5B).
Serum albumin was also investigated in hepatocyte-like cells at weeks 1-4. As it could be seen, the amount of production was higher over time which was significant in all weeks except for week one (p≤0.05). Also, the cells in passage 5 were checked and compared with passage 3; the proportion for the former indicated the significance in all weeks (p≤0.05) (Table 2, Graph 1), and for the latter showed significance for exposed cells between all weeks except for weeks 2-3, and for not exposed cells between all weeks except for weeks 1-2 as well as 2-3(data was not shown).
Discussion :
MSCs are important in research because of their application in both developmental biology and medicine such as tissue and organ repair 27. In the present study, WJ-MSCs were fibroblastic in appearance, and had the competence to form colony. A high frequency of colony forming units and short doubling time has been verified in these cells which may reflect their rather primitive nature 28.
Additionally, like most of the other previous works, high expression levels for CD105 and CD90 and low levels for CD45 and CD34 were demonstrated 29, which suggest that WJ-MSCs were of the MSC family and there was a negligible contamination with hematopoietic precursors.
The differentiation proficiency of umbilical cord blood 23 as well as bone marrow-derived 30 MSCs, adipose stromal cells 24, hepatic progenitor cells 25, and WJ-MSCs 31 into hepatocyte-like lineage was verified so far.
For promoting hepatocyte differentiation, FGF4 and HGF were used; the latter has the function on cytoskeletal rearrangement and inhibiting MSC proliferation through ras-ERK1/2 MAPK, and also antiapoptosis via PI3K/AKT pathway 27,32, but it cannot induce further maturation into hepatocyte-like cells 33. In this study, the improvement of differentiation potential was reported by using RA as an inducing factor; regarding cell diffrentiation with this factor, it has been suggested that gene properties, directly or indirectly and positively or negatively, influence wide varieties of culture conditions and cellular systems 34,35. Moreover, upregulation of genes through oxidase activation pathway and adhesion molecules as well as indirect effect on micro RNAs to regulate post-transcriptional mRNA processing has been reported 36. Furthermore, RA has a direct control on the cytoskeletal organization especially through cytokeratin phosphorelative modulation which in turn could restore the cell shape and promote cellular junctions and adhesions. These changes which led to the appearance of morphological features are more similar to those observed in vivo 32 and are compatible with our results. However, possible effects of RA on specific hepatocyte morphology and functions have not yet been known although the presence of two heterodimer families of nuclear receptors-RAR and RXR - has been confirmed in fetal and adult liver suggesting a probable role for RA in the control of differentiation and maturation of the cells 22,37. Taken together, our results and those of previous studies led us to propose this mechanism for RA in differentiation of WJ-MSCs.
There is evidence in favor of specific expression for hepatocyte markers including αFP and HNF1-α in differentiated cells under various hepatogenic media 30,32. In the current work, this expression was enhanced under induction of RA; however, it has been proposed that this factor has no or marginal effect on the mRNA levels of HNF-1α which in turn is a potent transcription factor for the albumin gene. Moreover, in a dose dependent manner, RA downregulates the secretion and gene expression of albumin in vivo 38. On the contrary, our data manifested the high levels of production for this protein and expression for the corresponding gene in RA exposed cells. The difference between our work and the others can be explained through two different variables of exposure time and dosage of RA.
Glycogen storage is tested as one important functional characteristic of hepatocyte. Our data revealed the significant rise in the process with RA treated cells. Consistent with these results, there is an evidence for inducing glycogen synthesis, as a marker for terminal hepatic differentiation by RA 37. Altogether, these findings are indicative of another essential role for RA in inducing the WJ-MSC terminal differentiation.
This work could introduce RA as a potent inducer of WJ-MSCs for differentiation into cells much similar to hepatocytes in morphology, function, and expression levels of specific genes in vivo which may open a window in development of successful cell-based therapies for various liver diseases in future clinical trials. It is worth noting that some limitations such as evaluation of further hepatocyte genes, in vivo application of differentiated cells as well as elucidating the contributing mechanism of RA in differentiation process were faced in this research.
Conclusion :
All in all, our findings suggest that RA is the potent inducing molecule in differentiation of WJ-MSCs toward hepatocyte-like cells; therefore, it may be considered as a promising factor for targeting liver disorder therapies.
Acknowledgement :
The authors are grateful for useful guidance and endless support of Anatomy Department of Tehran University of Medical Sciences in this project.
Conflict of Interest :
The authors have no conflicts of interest to declare.
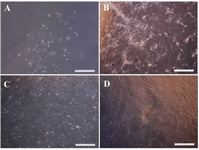
Figure 1. Morphologic features of Wharton's Jelly-Mesenchymal Stem Cells (WJ-MSCs). A) MSCs isolated from WJ with heterogeneous flattened fibroblast-like characteristics, B) cell expansion with homogenous form, C) and further expansion, D) morphology of colony forming cells (Invert microscope, Scale bar=100 µm).
|
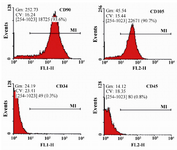
Figure 2. Flow cytometry analysis of human Wharton's Jelly-Mesenchymal Stem Cells (WJ-MSCs) for surface markers. The cells were positive for CD90 and CD105, but roughly negative for CD34 and CD45.
|
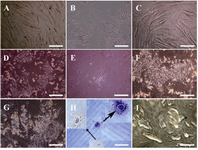
Figure 3. Hepatocyte-like cells morphological features. A) Serum free cells show no conspicuous growth alteration, B) short appendage flattened in cells exposed to RA as a contrast with C) not exposed cells after two weeks, D) after three weeks, polyhedral shapes were detected in exposed cells despite of E) not exposed cells, F) at the end of week’s four, more visible granules appeared in cells receiving RA than G) not receiving cells, H) morphology of two hexagonal HLCs stained with hematoxylin followed by I) further bile canaliculi-like (arrow) development in exposed cells. All observed with invert microscope. Scale bars for A, B and C=10 µm; for D, E, F, and G=100 µm; for H=20 µm; and for I=50 µm.
|
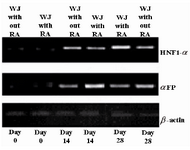
Figure 4. Expression pattern of hepatocyte-like cells specific markers. It is clear from the figure that the highest expression level for αFP was at day 14, but for HNF1-α at day 28. RA had the profound effects on expression levels of the two discussed markers. We can also see that the rates for cells at day 0 were not significant. WJ= Wharton’s jelly-mesenchymal stem cells; αFP=alpha-fetoprotein; and HNF-1α=hepatocyte nuclear factor-1α.
|
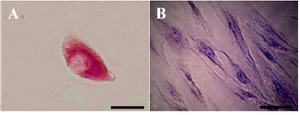
Figure 5. Periodic Acid-Schiff (PAS) staining for glycogen analysis. A) In the presence of Retinoic Acid (RA), glycogen storage in hepa-tocyte-like cells was at the high level but B) in the absence of this factor no storages was detected (Scale bar for A=30 µm, and for B=10 µm).
|
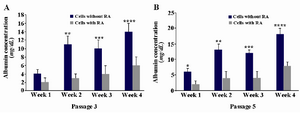
Graph 1. A and B) Albumin production assay in cells with or without retinoic acid (RA) exposure at passage 3 and 5, respectively. A) The high level of production was observed which was significant in all weeks except for week 1 (**:p≤0.003, ***: p≤0.02, ****: p≤0.008). B) The high level of production was observed which was significant in all weeks (*: p≤0.008, **: p≤0.005, ***: p≤0.003, ****: p≤0.001). Values are the means±SD of six samples.
|
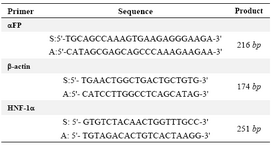
Table 1. Primer sequences designed for genes evaluated in this study: alpha-fetoprotein (αFP) and hepatocyte nuclear factor-1α (HNF-1α). Sense (S); Antisense (A); base pair (bp)
|
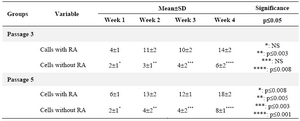
Table 2. Comparison of the mean±SD of four variables among the study groups for albumin detection
NS: Non-significant; RA=retinoic acid.
|
|