Expression of Recombinant Human Insulin-like Growth Factor Type 1 (rhIGF-1) in Escherichia coli
-
Iranpoor, Hamidreza
-
Department of Medical Biotechnology, Faculty of Advanced Technologies in Medicine, Golestan University of Medical Science, Gorgan, Iran
-
Omidinia, Eskandar
-
Genetics and Metabolism Research Group, Pasteur Institute of Iran, Tehran, Iran
-
Vatankhah, Venus
-
Faculty of Medical Sciences, Shahrekord University of Medical Sciences, Shahrekord, Iran
-
 Shahbazi, Majid
Cellular and Molecular Research Center, Taleghani Children Hospital, Gorgan University of Medical Sciences, Gorgan, Iran, Tel: +98 171 225 17 34, E-mail: shabazi.ma@gmail.com
Shahbazi, Majid
Cellular and Molecular Research Center, Taleghani Children Hospital, Gorgan University of Medical Sciences, Gorgan, Iran, Tel: +98 171 225 17 34, E-mail: shabazi.ma@gmail.com
-
Cellular and Molecular Research Center, Taleghani Children Hospital, Gorgan University of Medical Sciences, Tehran, Iran
Abstract: Background: Human insulin-like growth factor type 1 (hIGF-1) is a protein consisting of 70 amino acids (MW=7.6 kDa) and mainly synthesized by liver. Mecasermin (Trade name INCRELEX) is the synthetic form of the protein which is used as an effective treatment for particular disorders such as short stature, type 1 and 2 diabetes, and wound healing. Current study was aimed to investigate the expression of human insulin-like growth factor type1 in Escherichia coli (E. coli) BL21 (DE3) expression system in order to produce an active recombinant form of the protein.
Methods: For the purpose of the study, firstly codon optimization was done for hIGF-1 gene, using bioinformatics databases. Then, the gene was synthesized and inserted in pET-24a vector by a cutting strategy included NdeI and BamHI-HF enzymes. In the next step, gene was run in agarose gel and purified. The constructed expression cassette was transformed into E. coli BL21 (DE3) cells through CaCl2 heat shock method. Identification and confirmation of the transformed colonies were performed using screening PCR method. Synthesis of hIGF-1 was induced by IPTG. The expression in induced strains was analyzed by SDS-PAGE and western blotting techniques. Confirmation of cloning and IGF-1 expression cassette was carried out through genetic engineering procedures.
Results: Analysis of transformed E. coli strain with SDS-PAGE and western blotting techniques confirmed that gene was expressed in host cells. Molecular weight of the expressed protein was estimated to be 7.6 kDa.
Conclusion: hIGF-1 expression cassette for cloning and expression in E. coli was designed and the protein of interest was successfully induced and identified. In addition, E. coli BL21 (DE3) can be used as a suitable host for production of recombinant hIGF-1 and this technology has a potential to be localized.
Introduction :
Production of recombinant protein provides a suitable method for commercializing medical products 1. Another advantage of producing recombinant proteins is better safety in comparison with sampling of fluids from individual 2. hIGF-1 has a chemical structure similar to proinsulin 3,4. IGF-1 is also called somatomedin C, a small 70-amino acid polypeptide with a molecular weight of 7649 kDa 5. This protein is single chain and has three disulfide bonds. IGF-1 is synthesized by many tissues and is secreted from liver as an endocrine hormone which is transmitted to other tissues. This protein is also secreted by other tissues, including cartilage tissue and acts as a paracrine hormone locally 6. IGF-1 is responsible for cell differentiation, transformation, suppression of apoptosis, cell cycle progression, cell proliferation and differentiation. This protein stimulates the systemic growth of body and has a growth-enhancing effect on every part of the body, especially on skeletal muscle, cartilage, bone, liver, kidneys, nerves, skin, hematopoietic cells and lung 7. In addition to insulin-like effects, IGF-1 can also regulate cell growth and development, especially in nerve cells via cellular DNA synthesis 8. To increase the height of children with growth hormone deficiency 9 and also to treat people who suffer from IGF-1 deficiency such as Laron syndrome or Laron-type dwarfism (short stature syndrome), recombinant IGF-1 is prescribed 8. The recombinant human IGF-1 (Trade name: INCRELEX) was approved by FDA of the United States of America in 2006 and also received the EP confirmation in 2007 10. Due to the high throughput of recombinant DNA techniques, the commercial production of peptides and proteins in bacterial cells is performed this way 11. One of the microorganisms which can be used for cloning and expression is a special strain of Escherichia coli (E. coli). This expression system has many advantages such as simple control of gene expression, protein efficiency (up to 50% of total cell protein), having a cloning vector and easy culture 11,12. Our main goal was cloning and expression of recombinant human insulin- like growth factor type 1.
Materials and Methods :
Design and synthesis of genes: Human insulin-like growth factor type 1 is a non-glycosylated protein and the best expression system for this protein is E. coli. Thus, IGF-1 gene was optimized for this bacterium. This codon optimization was done since IGF-1 is a human protein, while the chosen host to produce the recombinant form of protein is E. coli which is a prokaryotic cell. In essence, the codon usage pattern in E. coli is different from natural host that is human. Hence, in order to homogenize the codon usage pattern of the desired protein sequence and expression in the host and finally overexpression of gene, codon optimization and gene synthesis were performed by
GenScript Corporation. IGF-1 sequence is shown below:
|
|
IGF-1 gene (225 bp)
|
|
Before
optimization
|
CATATGGGTCCGGAAACCCTGTGCGGTGCTGA
ACTGGTTGACGCTCTGCAGTTCGTTTGCGGTGA
CCGTGGTTTCTACTTCAACAAACCGACCGGTTA
CGGTTCTTCTTCTCGTCGTGCTCCGCAGACCGG
TATCGTTGACGAATGCTGCTTCCGTTCTTGCGA
CCTGCGTCGTCTGGAAATGTACTGCGCTCCGCT
GAAACCGGCTAAATCTGCTTGAGGATCC
|
|
After
optimization
|
CATATGGGCCCGGAAACCCTGTGTGGTGCGGA
ACTGGTGGATGCCCTGCAATTCGTGTGTGGTGA
CCGTGGCTTTTACTTCAACAAACCGACCGGCTA
TGGTAGCTCTAGTCGTCGCGCACCGCAGACCG
GCATTGTGGATGAATGCTGTTTTCGTTCCTGCG
ACCTGCGTCGCCTGGAAATGTACTGTGCGCCGC
TGAAACCGGCGAAAAGCGCCTGAGGATCC
|
|
|
|
NdeI restriction site
|
CATATG
|
|
BamHI restriction site
|
GGATCC
|
|
Stop codon
|
TGA
|
|
Then, this gene was sub-cloned into pUC18 plasmid. Protein sequence (70 aa) is shown below:
GPETLCGAELVDALQFVCGDRGFYFNKPTGYGSSSRRAPQTGIVDECCFRSCDLRRLEMYCAPLKPAKSA
Cloning of human insulin-like growth factor type 1 gene in E. coli DH5α: Cloning vector pUC18 and IGF-1 gene were purchased from GenScript (USA). Initially, optimized IGF-1 gene was sub-cloned into pUC18. Cloning vector was transformed into E. coli DH5α competent bacteria in order to replicate the plasmid. After the transformation, bacteria were cultured in LB Agar medium containing IPTG, X-Gal and ampicillin (50 µg/ml final concentration) and were incubated for 16 hr at 37°C. Then, using white-blue screening of colonies, transformed bacteria that formed white single colonies were picked up by loop and cultured in 100 ml SOC medium and incubated in shaker incubator for 12 hr at 37°C. Next, bacterial sediment was prepared using centrifuge, and replicated plasmids were extracted by plasmid extraction kit (Qiagen, Netherlands). For plasmid confirmation, it was run in 1% agarose gel electrophoresis. Extracted plasmid concentration was 100 ng/µl.
Enzymatic digestion of pUC18 cloning vector and pET-24a expression vector: Firstly, this plasmid was digested with two restriction enzymes including NdeI and BamHI-HF (BioLabs (UK)), for separation of insulin-like growth factor type 1 gene from pUC18 cloning vector. Then, it was run on 1% agarose gel electrophoresis and purified with gel extraction kit (Qiagen, Netherlands) in order to extract the digested gene. Final concentration of obtained gene was 14 ng/µl. pET-24a was also digested by these two restriction enzymes and prepared for ligation.
Ligation of IGF-1 gene into pET24-a expression vector: The equation below can be used to calculate the quantities of DNA necessary for a particular ratio:
|
Vector (ng)×size of insert (kb)
|
× Molar ratio of insert/vector)=insert (ng)
|
|
Size of vector (kb)
|
According to BioLab protocol, the best molar ratio is 3:1. 30 µg of expression vector with 1.27 µg of IGF-1 gene and 5 µl of T4 DNA Ligase (BioLabs (UK)) were used for ligation.
Analysis of transformants by colony screening: Colonies can be screened for inserts without plasmid preparation, by direct colony PCR using vector-specific primers. To verify ligation between the vector and insert, a ligation reaction can be analyzed directly by PCR using vector-specific primers. For pET-24a, appropriate primers for screening by colony PCR were T7 Promoter Primer (TAATACGACTCACTATAGGG) and T7 Terminator Primer (GCTAGTTATTGCTCAGC GG). According to pET manual from Novagen Company, a colony was picked up from an agar plate using a sterile toothpick. Colonies chosen were at least 1 mm in diameter and an attempt was made to get as many cells as possible. Then, each colony was transferred to a 0.5 ml micro-tube containing 50 μl of sterile water. Vortex was done to disperse the cells. Then, the micro-tube was placed in boiling water or a heat block at 99°C for 5 min to lyse the cells and denature DNases. Next, it was centrifuged at 12000×g for 1 min to remove cell debris. After that, 10 μl of the supernatant were transferred to a fresh 0.5 ml micro-tube to do PCR and it was left on ice until use. Then, a master mix was prepared for colony PCR by assembling the following components: 1 µl dNTP mix 10 mM, 1 µl upstream primer, 1 µl downstream primer with 5 pmol concentration, 5 µl 10´buffer with MgCl2, 0.25 µl Taq DNA polymerase, and 31.75 µl nuclease free water. Next, 40 μl of the master mix were added to each 10 μl sample and were mixed gently and the samples were put in a thermal cycler. The processes in the thermal cycler for 35 cycles included: 1 min at 94°C, 1 min at 55°C, 2 min at 72°C, 6 min final extension at 72°C. Then, 10-25 μl of the product of colony PCR were loaded per lane on a 1% agarose gel. A strong band was appeared that had a size corresponding to the total number of bases of the region between the two primers.
pET-24a transformation into E. coli BL21(DE3) expression system and expression of rhIGF-1: pET-24a expression vector and E. coli BL21(DE3) were purchased from Pasteur Institute (Iran). Expression vector carrying the desired gene, was transformed into E. coli BL21 (DE3). Then, it was cultured on LB Agar medium and incubated for 6 hr at 37°C. Next, single colonies were picked up from plate, each transferred to 100 ml LB Agar containing kanamycin (30 µg/ml final concentration) medium and incubated in shaker incubator at 37°C. Then, when OD600 reached 2, IPTG (1 mM) was added and it was incubated again for 5-6 hr. Thus, protein expression was completed and IGF-1 protein was produced as inclusion bodies in bacterial cytoplasm. Finally, whole culture was centrifuged at 10000´rpm.
Disruption of bacterial cell and cell lysis: After centrifugation, the sediment was weighed to obtain 0.2 g. Then, it was dissolved in DTT (400 ml) and homogenized using SOAVI homogenizer (Parma, Italy) (4 times with 1200 bar pressure). After the breakdown, the solution was centrifuged at 11000 rpm for 5 min. The precipitate was weighed to obtain 0.1 g. Then, the precipitate was dissolved in washing buffer (Triton (´100) 0.5% , Tris (50 mM), EDTA (5 mM), and DTT (1 mM)) and incubated for 40 min at room temperature. Then, this solution was centrifuged at 15000 rpm for 15 min and precipitate was dissolved in 100 µl WFI (water for injection).
Analysis of recombinant protein expression: Gene expression was evaluated in the induced strain by SDS-PAGE and western blotting techniques. SDS-PAGE was done using 20% poly-acrylamide gel and samples were stained by bromophenol blue. Finally, western blotting was done using primary polyclonal antibody against IGF-1 (Abcam Corporation, USA) and IgG secondary antibody was conjugated to HRP (Abcam Corporation, USA). Western blotting analysis was performed using anti-IGF-1 polyclonal antibody as the primary antibody, goat anti-rabbit antibody (IgG-HRP) as the secondary antibody and DAB, 50X. Protein bands on SDS-PAGE were transferred to nitrocellulose membrane in order to identify the production of hrIGF-1 in E. coli BL21 (DE3). The first incubation of membrane was accomplished with bovine serum albumin (BSA) 3% (w/v) in tris-buffered saline and PBS solutions for 90 min to block unspecific bands on the membrane. The second incubation was done by anti-IGF-1 polyclonal antibody added at a dilution of 1:500 in PBS solution. Anti-IgG at a dilution of 1:1000 in PBS solution was used in third incubation. Subsequently, incubation was performed with diaminobenzidine solution (DAB) for 20 min (0.5 mg/ml DAB, 0.1% H2O2).
Results :
Cloning of IGF-1: In order to confirm plasmid (pUC18) replication in E. coli DH5α, electrophoresis on 1% agarose gel was done following the extraction process. The sizes of pUC18 and IGF-1 gene were 2686 and 219 bp, respectively. NdeI site and BamHI restriction sites were located on nucleotide 183 and 429, respectively. Size of pUC18 digested with NdeI and BamHI restriction enzymes was 2440 bp. Therefore, size of pUC18-IGF-1 was 2659 bp. The results (presence of 2659 bp band) indicated that pUC18 plasmids were replicated successfully.
Enzymatic digestion of pUC18 cloning vector and pET-24a expression vector: After digestion of the pUC18 plasmid with two restriction enzymes (BamHI-HF and NdeI), electrophoresis was done on 1% agarose gel and a 225 bp band was detected which indicated that IGF-1 gene was sub-cloned into pUC18 plasmid successfully (Figure 1).
Colony PCR: In order to confirm ligation of the gene into expression plasmid (pET-24a), colony PCR was run. As it is shown (Figure 2), lane 2 contained IGF-1 sequence and the region was located between two primers (425 bp band).
SDS-PAGE: After confirmation of IGF-1 ligation into pET-24a, analysis of protein expression was done using SDS-PAGE technique. As it is shown (Figure 3), there was a 7.6 kDa band which indicated the expression of rhIGF-1 gene.
Western blotting: Western blotting results also indicated the expression of rhIGF-1 by representing a 7.6 kDa band on nitrocellulose membrane (Figure 4).
Discussion :
In this study, E. coli BL21 (DE3) was used as a cloning host to express human insulin-like growth factor type 1. Human insulin-like growth factor type 1 is an effective therapeutic protein for treatment of patients suffering from short stature, diabetes type 1 and 2 as well as wound healing 5. So far, it has not been produced in Iran. So, the current study has made the first step for the localization of the technology of recombinant IGF-1 production in Iran. However, cloning and expression of IGF-1 only in E. coli were investigated. A variety of hosts are used for production of therapeutic proteins such as yeast 13,14 and mammalian cells 6. However, due to low expression levels and post-translational modifications of these hosts, E. coli is a more suitable expression host for many cases. Since IGF-1 is a non-glycosylated protein, E. coli can be a suitable host for large-scale production of it. In addition, E. coli is capable to produce high amounts of the recombinant protein. In other studies, different types of E. coli have been used, but E. coli BL21(DE3) was used for this study because this strain has been engineered for high level expression of protein for reasons stated above 15. The prokaryotic expression systems of IGF-1 such as E. coli W3110 7, E. coli 294 2, and E. coli HB101/PLSD1 16 have been used but E. coli BL21 (DE3) derivation of B834 was selected in this paper because it contains T7 gene endogenously in its bacterial chromosome and produces T7 RNA polymerase which in turn can increase the production of recombinant protein. Moreover, this strain possesses a deficiency in the lon protease and lacks ompT outer membrane protease. This deficiency prevents the recombinant protein from damage. Our priority to choose the host for protein expression was the high level of the production of the recombinant protein. The E. coli cytoplasm is regarded as the first choice in protein production owing to its high efficiency 7,17. However, inclusion body formation occurs as the result of protein over-expression in cytoplasm 18. Inclusion bodies can be an advantage for purification since 1) they are easily isolated by centrifugation to yield highly concentrated and relatively pure protein, and 2) inclusion body formation protects the protein from proteolytic attack. Moreover, toxic proteins may not inhibit cell growth when present in inactive form as inclusion bodies 19. Human IGF-1 has been expressed as inclusion bodies in E. coli cytoplasm.
In this report, IGF-1 production in E. coli BL21 (DE3) under control of T7 promoter was investigated. To over-produce IGF-1, pET-24a plasmid containing the powerful T7 promoter was used. Given the importance of rhIGF-1, efforts were made to clone and express it in E. coli BL21(DE3). Also, identification of the produced recombinant protein was specifically confirmed by SDS-PAGE and western blotting.
Conclusion :
In this study, the expression cassette for expression of human insulin growth factor type1 in Escherichia coli was designed and the protein was identified successfully. E. coli BL21(DE3) can be used as a suitable host for the production of recombinant human insulin-like growth factor type 1 and this technology has the potential to be localized in Iran.
Acknowledgement :
This project was financially supported by the Golestan University of Medical Sciences (Grant no. 920919150).
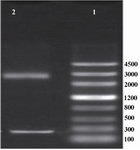
Figure 1. Electrophoresis of the products of pUC18 plasmid. Lane 1 contained DNA ladder (Genscrip (USA)), lane 2 contained 2 bands representing pUC18 and IGF-1
|
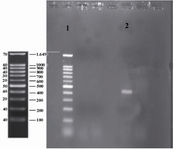
Figure 2. Colony PCR of IGF-1 sequence integrated into pET-24a. Lane 1 contained DNA Ladder (Sinagene (Iran)) and lane 2 contained IGF-1 sequence or the region located between two primers (425 bp band).
|
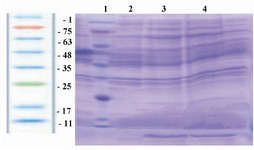
Figure 3. SDS-PAGE for analysis of the expression of IGF-1 protein in E. coli BL21 (DE3). Lane 1(protein ladder) contained protein ladder (Sinagene, Iran), lanes 3 and 4 contained a 7.6 kDa band, representing the expression of rhIGF-1 protein induced by IPTG, and lane 2 representing the pattern of transformed BL21 under-un-induction condition (without IPTG).
|
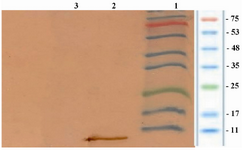
Figure 4. Analysis of the expression of rhIGF-1 using western blotting technique. Lane 1 contained protein marker (Sinagene, Iran), lane 2 contained rhIGF-1 protein and lane 3 representing the pattern of transformed BL21 under un-induction (without IPTG).
|
|