The Immunosuppressive Activity of Amniotic Membrane Mesenchymal Stem Cells on T Lymphocytes
-
Alikarami, Fatemeh
-
Blood Transfusion Research Center, High Institute for Research and Education in Transfusion Medicine, Tehran, Iran
-
 Yari, Fatemeh
Blood Transfusion Research Center, High Institute for Research and Education in Transfusion Medicine, Tehran, Iran, Tel: +98 21 82052238, E-mail: f.yari@ibto.ir
Yari, Fatemeh
Blood Transfusion Research Center, High Institute for Research and Education in Transfusion Medicine, Tehran, Iran, Tel: +98 21 82052238, E-mail: f.yari@ibto.ir
-
Blood Transfusion Research Center, High Institute for Research and Education in Transfusion Medicine, Tehran, Iran
-
Amirizadeh, Naser
-
Blood Transfusion Research Center, High Institute for Research and Education in Transfusion Medicine, Tehran, Iran
-
Nikougoftar, Mahin
-
Blood Transfusion Research Center, High Institute for Research and Education in Transfusion Medicine, Tehran, Iran
-
Jalili, Mohammad Ali
-
Blood Transfusion Research Center, High Institute for Research and Education in Transfusion Medicine, Tehran, Iran
Abstract: Background: Mesenchymal Stem Cells (MSCs) are isolated from different sources like placenta. The placenta and its membranes like Amniotic Membrane (AM) are readily available and easy to work with. There is only limited knowledge on the immunomodulatory properties of human Amniotic Membrane-derived Mesenchymal Stem Cells (hAM-MSCs). The aim of this study was to survey the suppressive activity of hAM-MSCs on T lymphocytes in vitro.
Methods: Human AMs were obtained after caesarean section births from healthy women. After enzymatic digestion, cells were cultured and hAM-MSCs were obtained. In addition, human T lymphocytes were isolated and co-cultured with hAM-MSCs for 72 hr in the presence or absence of phytohemagglutinin (PHA). Subsequently, proliferation of T cells was analyzed using BrdU and subsequently flow cytometry technique. Besides, the production of IL-4 and IFN-γ was examined by ELISA method. Additionally, the expression of activation markers (CD38, HLA-DR) was studied on T lymphocytes by flow cytometry technique.
Results: It was revealed that hAM-MSCs could significantly suppress the proliferation of T lymphocytes (p≤0.01) and significantly decrease the production of IFN-γ by T cells (p<0.05). hAM-MSCs also down regulated the expression of activation markers on the surface of T lymphocytes, CD38 and HLA-DR. The difference was significant between the case and control samples (p<0.05). All the comparisons were carried out between the case (Tcell+PHA+hAM-MSCs) and control (Tcell+PHA) groups.
Conclusion: In conclusion, hAM-MSCs could inhibit the (mitogen-activated) T cells even in the absence of blood monocytes. Besides, hAM-MSCs-mediated inhibition of T lymphocytes was combined with down regulation of activation markers.
Introduction :
Mesenchymal Stem Cells (MSCs) are multipotent non-hematopoietic progenitor cells which have high potential for differentiation into much different types of tissues 1-5. One of the important properties of these cells is their immunomodulatory effects. MSCs can inhibit different types of immune cells such as T 6-8, B 9, NK 10 and dendritic cells. T-cells are the most important cells in adaptive immune system. Dysregulation of T-cell function can lead to autoimmune or allergic diseases. Moreover, the immune responses mediated by T-cells have important role in Graft-Versus-Host Disease (GVHD) and transplant rejection. Suppression of T-cells induced by MSCs has been reported by several studies 6-8. The osteogenic differentiation of hAM-MSCs has been detected in a significantly high number of these cells 11. Recently, MSCs have been used to avoid graft-versus-host disease after allogeneic hematopoietic stem cell transplantation 12,13. These cells can improve experimental auto-immune encephalomyelitis in a model of multiple sclerosis and enhance the survival in a model of allotransplantation in baboon 14.
The common source for MSCs is still Bone Marrow (BM). But since Bone Marrow-derived MSCs (BMSCs) constitute a small population (under 0.1% of nucleated cells), providing MSCs from other sources will be valuable 15,16. At present, the current and available source of MSCs is placenta and its membranes 16-18. In this research, hAM-MSCs were selected because of their easily accessible source and the existence of fewer reports related to these cells. Amniotic membrane is a thin semitransparent tissue which may have a role in immunological tolerance since it is located between maternal and fetal interface 16,17. The immunomodulatory properties of hAM-MSCs are not completely defined. In this regard, the inhibitory effects of hAM-MSCs on T-cells proliferation and activation were evaluated in the presence or absence of the mitosis inducer, Phytohaemagglutinin (PHA).
Materials and Methods :
Isolation of placenta-derived mesenchymal stem cells, hAM-MSCs: Human placentas were collected during caesarean section births of healthy women after approval of the local ethics board and obtaining informed consent. Samples of AM were collected in sterile conditions by a specialist at Milad Hospital in Tehran and transferred in a tube containing phosphate-buffered saline (PBS, ICN Biomedicals, Ohio, USA) and antibiotics. Under sterile condition, the AM was isolated from placenta and cut into small pieces. The pieces were washed several times with PBS containing antibiotics (Cinagen, Karaj, Iran) and digested with collagenase type I (Gibco, Grand Island, USA) in a shaking incubator at 37°C for 1-2 hr. To clear cell suspensions of unwanted materials, they were filtered and centrifuged at 750 g for 5 min. The cells were introduced into the DMEM-Low Glucose medium (Sigma Aldrich, Gillingham, Wisconsin, USA) contained FBS (Invitrogen, Camarillo, California, USA) at a final concentration of 10%, Penicillin-Streptomycin (10,000 units penicillin and 10 mg streptomycin/ml) and were seeded into T75 flasks (Nunc, Roskilde, Denmark). The flasks were placed in an incubator at 37°C in humidified atmosphere with 5% CO2. hAM-MSCs at passage 3 were irradiated with gamma rays from a Cs-137 source (30 Gy or 3000 rad) and used for co-culture with T-cells.
Characterization of placenta-derived mesenchymal stem cells: The expression of MSCs-specific surface markers was studied on the cultured hAM-MSCs after passage 3. The cells were separately stained with a set of fluorochrome-conjugated antibodies [CD29-FITC, CD105-FITC, CD44-FITC, CD73-PE, CD34-PE, CD166-PE and CD45-FITC (all from Dako, Produktionsvej, Denmark)] for 45 min at 4°C. After the washing step, the cells were analyzed by flow cytometry technique (Partec 3, USA).
Differentiation ability of hAM-MSCs in vitro: The ability of hAM-MSCs to differentiate into osteoblast cells was surveyed using mesenchymal stem cell osteogenesis kit (Chemicon international, USA). Briefly, vitronectin and collagen each in the concentration of 12 μg/ml were added into a 24-well culture plate and incubated at room temperature for 24 hr. The wells were washed by PBS and hAM-MSCs (6×104 cells) were inserted in the presence of DMEM-Low Glucose medium. The cells were incubated at 37°C, 5% CO2 for 24 hr. The differentiation was induced by incubation of hAM-MSC with osteogenic complete medium for 14 days. This medium consisted of ascorbic acid, dexamethasone, and 6-glycerol phosphate. After 14 days, the cells were stained by Alizarin red-S and the red calcium deposits were visualized by microscopic examination.
Isolation of human T lymphocytes: Peripheral blood mononuclear cells were separated with density gradient centrifugation using Ficoll-Paque (Amersham bio sciences, Uppsala, Sweden). Peripheral blood mononuclear cells were incubated with monoclonal antibodies against CD16, CD19 and CD14 (all from Abcam, Cambridge, USA) at 2-8°C for 30 min for removing NK-cells, B-cells and monocytes, respectively. Cells were washed with PBS and treated with dynabeads® goat anti-mouse IgG (Invitrogen Dynal AS, Oslo, Norway) based on the manufacturer's instructions. The cell suspension and magnetic beads were incubated at 2-8°C for 30 min again with mild agitation. Using a magnetic particle concentrator, different cells were removed and only T cells remained. Finally, the viability of T cells was estimated by trypan blue exclusion method and the purity of them was analyzed using anti-CD3 (Abcam, Cambridge, USA) with flow cytometry technique.
Co-culture of hAM-MSCs and T cells: hAM-MSCs were irradiated with Caesium-137 (30 Gy) for 20 min to prevent cell proliferation ability. Variable numbers of hAM-MSCs, 5×103, 10×103 and 20×103, were introduced into the wells of a 96 well cell culture plate. T cells (106 cells) were also added to each well in the presence or absence of 1 μg/ml PHA ((Invitrogen, Camarillo, California, USA) and incubated for 72 hr in a cell culture incubator (the total reaction volume was 250 μl). All the choices for the co-culture of hAM-MSCs and T cells were as below:
- Tcell+PHA+hAM-MSCs (5×103 cells)
- Tcell+PHA+hAM-MSCs (10×103 cells)
- Tcell+PHA+hAM-MSCs (20×103 cells)
- Tcell+hAM-MSCs
- Tcell (Negative control)
- Tcell+PHA (control T cells)
Cell proliferation test: T lymphocyte proliferation was evaluated by using the thymidine analog, 5-bromo-2-deoxyuridine (BrdU) with Pharminigen Flow kit (BD Biosciences, San Diego USA) according to the manufacturer's recommendations. Briefly, after 68 hr of co-culture of hAM-MSCs and T-cells, 10 μl of BrdU (10 mg/ml) was added for 1 ml of culture medium and incubation continued for another 4 hr. The cells were fixed and permeabilized with BD Cytofix/Cytoperm Buffer. Then, the cells were treated with DNase to unwind the DNA and help the anti-BrdU antibody access the incorporated BrdU. Afterwards, they were stained with fluorescent antibody against BrdU. For staining total DNA, 7-AAD was used. The 7-AAD population was gated and BrdU positive cells were analyzed as an index for replicating cells by flow cytometry. It should be noted that an aliquot of BrdU-unlabeled cells were used as the negative control.
Measurement of IL-4 and IFN-γ: After 72 hr of co-culture, the supernatants of the culture media were taken and centrifuged. ELISA method was used to determine the amounts of IL-4 and IFN-γ in the supernatants by using human IL-4 ELISA kit (Cambridge, USA) with the sensitivity of <0.5 pg/ml and human IFN-γ ELISA kit (Invitrogen, Camarillo, California, USA) with the sensitivity of <4 pg/ml.
Assessment of CD38 and HLA-DR expression: The activation markers of T cells were analyzed after 72 hr co-culture of hAM-MSCs and T cells by flow cytometry. The content of wells was taken and centrifuged. The pellet was suspended in PBS and treated with phycoerythrin-conjugated (PE) anti-CD38, (United States Biological, Salem, Massachusetts USA) or anti-HLA-DR (Abcam, Cambridge, USA) for 45 min at 4°C. The reaction for HLA-DR was carried out in two steps, in which F(ab')2 anti-mouse IgG PE (eBioscience, San Diego, USA) was used in step 2 for 25 min. To ensure the specificity of the results, nonspecific antibody background binding was determined with the appropriately labeled isotypic control immunoglobulin IgG. The cells were finally analyzed by flow cytometry technique. The total number of samples in all the experiments was 3 (N=3).
Statistical analysis: Paired T-test with SPSS 16.0 software was used to compare the results of this experiment. The p<0.05 was considered statistically significant.
Results :
Characterization of hAM-MSC: Surface markers of MSCs were distinguished on hAM-MSC by flow cytometry technique. hAM-MSCs were positive for CD105 (92.7±0.9%), CD44 (93.3±1.3%), CD29 (93.5±2%), CD73 (92±4.1%), CD90 (90±3.2%), and CD166 (89.1±2.1%), but were negative for CD45 (4.1±0.08%) and CD34 (3.2±0.1%). The flow cytometry plots were shown in figure 1.
Differentiation ability of hAM-MSC: Osteogenic differentiation ability of isolated hAM-MSC was confirmed by positive Alizarin red S staining. By using this dye, red calcium deposits could be seen in the differentiated cells (Figure 2). Control and treated cells were tested alternately. Control cells that were cultured in the absence of the differentiation medium did not display red calcium deposits.
Inhibition of T cell proliferation following co-culture of T cells with hAM-MSC: It was revealed that the purity of CD3+T cells was 85±2%. The purified T cells were stimulated with PHA and co-cultured with hAM-MSC for 68 hr. After 4 hr incubation with BrdU, the proliferation of T lymphocyte was analyzed by flow cytometry. T cell proliferation was significantly inhibited following co-culture of T-cell/hAM-MSC (p≤0.01). As it is evident in table 1 and figure 3, at higher concentrations of hAM-MSC (20×103 cells), higher suppression of proliferation was seen.
Evaluation of IFN-γ and IL-4 production by T lymphocytes: IL-4 and IFN-γ were measured in the supernatant of the culture medium to evaluate the effects of hAM-MSC on the activity of the T cells. As it could be seen in table 1 and figure 4A, the inhibitory effects of hAM-MSC on the production of IL-4 were not remarkable and the differences were not significant between each bar and T cell+PHA (control T cells) group. Nevertheless, in different densities of hAM-MSC, the production of IFN-γ significantly decreased compared to T cell+PHA (control T cells) group (Table 1 and Figure 4B).
Changes in the activation markers of T cells: The expression of activation markers on T cells after co-culture with different densities of hAM-MSC for 72 hr. hAM-MSC caused a reduction in the expression of Tcell activation markers, CD38 and HLA-DR. As it could be seen in figures 5A and 5B, at higher densities of hAM-MSC (20×103 cells), the maximum inhibitory effects of hAM-MSC were observed for the expression of CD38 and HLA-DR (p≤0.01).
Discussion :
At present, MSCs have received attention for their immunomodulatory properties. Several studies have demonstrated the inhibitory effects of BMSCs on T lymphocytes. Additionally, the inhibitory effects of BMSCs have been investigated on immune cells such as NK and dendritic cells 19. BMSCs are capable of altering the outcome of the immune cell responses by different mechanisms. More studies have shown that the immunosuppressive effects were associated with two mechanisms; first, soluble factors such as indoleamine 2,3 dioxygenase (IDO), prostaglandin E2 (PGE2), transforming growth factor beta (TGF-ß) and second, cell-cell contact 20-23.
In this study, human amniotic membrane as an accessible and valuable source of MSCs was the main focus. It was showed that 1) ex vivo-expanded hAM-MSCs had immunosuppressive effects on T lymphocyte proliferation induced by the lectin PHA, 2) hAM-MSCs could affect production of Th2 cytokine, IL-4 and Th1 cytokine, and IFN-γ, 3) hAM-MSCs suppressed the expression of activation markers (CD38 and HLA-DR) on T cells, 4) the inhibitory effects of hAM-MSCs were associated with the density of these cells.
Recently, few studies have shown that hAM-MSCs exert inhibitory effects similar to MSCs derived from BM or adipose tissue. For example, Kang et al. reported that Mitogen-induced Peripheral Blood Mononuclear Cells (PBMC) proliferation was suppressed by hAM-MSCs in a dose-dependent manner as well as hAM-MSC culture supernatant 18. Kang studied the inhibitory effects of hAM-MSCs on PBMCs whereas, in this study, the effects of hAM-MSCs on purified T lymphocytes were investigated.
It was found that hAM-MSCs decreased the production of IL-4 and IFN-γ cytokines but the differences were only significant for IFN-γ. There were several reports about the suppression of cytokine production following co-culture of T lymphocytes and MSCs. Aggarwal et al. reported that human BMSCs caused Th1 cells to decrease IFN-γ and the Th2 cells to increase secretion of IL-4 19. Besides, Yoo et al explained that levels of IFN-γ and TNF-α secreted from activated T cells were significantly reduced when co-cultured with each type of MSCs 1. In addition, Si et al reported that in co-culture of BMSCs with T cells, the expression of IFN-γ decreased while expression of IL-4 increased 24. However, Ueta et al showed that allo-reactive T cell synthesis of both Th1 (IL-2 and IFN-γ) and Th2 (IL-6 and IL-10) type cytokine significantly decreased in the presence of amniotic membrane (AM) when AM was added to MLR culture well 25. Different reports show conflicting results related to IL-4 changes after co-culture of T cells with hAM-MSCs. An inhibitory effect for hAM-MSCs was observed in the production of IL-4 by T cells. But the difference was not significant. Perhaps, performing the experiment under other densities of hAM-MSCs could change the obtained results for IL-4.
It was showed that ex vivo-expanded hAM-MSCs had immunosuppressive effects on T cell proliferation induced by the lectin PHA. Dazzi et al reported that hAM-MSCs were not constitutively immunosuppressive but they require a "licensing" step provided by molecules of acute phase inflammation, like IFNγ and TNF-α, or toll-like receptor (TLR) ligands 26. Taking the previous studies and our findings together, it is suggested that MSCs exerted their immunosuppressive effects when were placed in an inflammatory environment. This condition was generated using activation of T cells with PHA.
In the present investigation, it was also demonstrated that the expression of CD38 and HLA-DR was reduced on PHA-stimulated T cells after co-culture with hAM-MSCs. Our studies were in line with the report of Le Blanc et al. They described the inhibitory effects of mesenchymal stem cells on the expression of CD25 (interleukin-2 receptor) and CD38 on PHA activated T cells 6. Furthermore, Groh et al also demonstrated that BMSCs -mediated inhibition of T cells was associated with the down regulation of their activation markers, CD25, CD38 and CD69 8. In our study, in addition to CD38, the expression of HLA-DR was also considered as an activation marker for T cells.
It was also found out that the inhibitory effects of hAM-MSCs on T cells were dose dependent. Similarly, Kang reported that hAM-MSCs inhibited ConA-induced proliferation of PBMC in a dose-dependent manner 18. Furthermore, Klyushnenkova explained that MSCs suppressed cell proliferation in MLR culture and suppression was dose-dependent 27.
Our work on hAM-MSCs had several virtues; firstly, comparing with BMSCs, there were only limited reports about the immunosuppressive effects of hAM-MSCs. Secondly, in the mentioned studies, the inhibitory effects of hAM-MSCs were examined through their effects on PBMCs 18,26 whereas the effects of hAM-MSCs on purified T lymphocytes were investigated in this research. Thirdly, unlike the study of Groh et al who showed that CD14+monocytes from blood activated BMSCs to secrete inhibitory molecules that lead to inhibition of T cells 8, in this study, it was demonstrated that inhibition of T lymphocyte by hAM-MSCs did not need the interaction between blood monocytes and the MSCs. This observation might imply a different mechanism of action for hAM-MSCs compared to BMSCs.
Conclusion :
In conclusion, hAM-MSCs could prevent the proliferation and activation of (mitogen-activated) T cells even in the absence of blood monocytes. Besides, hAM-MSCs-mediated inhibition of T lymphocytes was associated with down regulation of activation markers, CD38 and HLA-DR. Further studies can be carried out to elucidate the possible therapeutic effects of culture-expanded hAM-MSCs like prevention of GVHD or its use in regenerative medicine.
Acknowledgement :
This study was the result of a thesis financially supported by Blood Transfusion Research Center, High Institute for Research and Education in Transfusion Medicine, Iranian Blood Transfusion Organization, Tehran.
Fatemeh Alikarami performed the research, Fatemeh Yari and Naser Amirizadeh supervised the project, Mahin Nikougoftar helped in flow cytometry technique, Mohammad Ali Jalili helped in data collection. All authors read and approved the final manuscript.
Conflict of Interest :
There is no conflict of interest to declare.
Financial support :
Iranian Blood Transfusion Organization, Research Center.

Figure 1. Flow cytometry plot. hAM-MSCs were prepared and their surface markers were assessed by flow cytometry technique. A) hAM-MSC were gated, B-C) isotype controls, D-E) hAM-MSCs were positive for CD29 and CD166, respectively. F-I) hAM-MSCs were also positive for CD105, CD73, CD44 and CD90, respectively. J-K) hAM-MSCs were negative for CD45 and CD34, respectively.
|
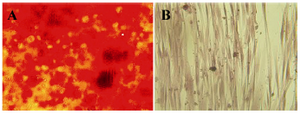
Figure 2. The in vitro osteogenic differentiation of hAM-MSC. A) The osteogenic differentiation of hAM-MSC was followed by Alizarin red S staining. B) Red calcium deposits could not be seen in negative control that was cultured in the absence of the differentiation medium.
|
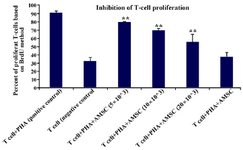
Figure 3. The inhibitory effects of hAM-MSCs on the proliferation of T cells. T cells (1×106) were co-cultured with different densities of irradiated hAM-MSCs in the presence or absence of PHA in the final volume of 250 μl for 72 hr. Each bar was compared with the T cell+PHA (control T cells) group. Data were presented as the mean ±SD of four independent experiments **p≤0.01.
|
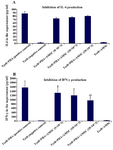
Figure 4. Cytokine levels in the supernatant of the co-culture medium of hAM-MSCs and Tcells in the presence or absence of PHA. A) Production of IL-4 and B) IFN-γ was detected using ELISA method. Each bar was compared with T cell+PHA (control T cells) group. Data were presented as the mean±SD of four independent experiments *p<0.05, ** p≤ 0.01.
|
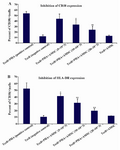
Figure 5. T cells were co-cultured with hAM-MSCs for 72 hr in the presence or absence of PHA. The inhibitory effects of hAM-MSCs on the expression of A) CD38 and B) HLA-DR on the surface of T cells were shown. Each bar was compared with T cell+PHA (control T cells) group. Data were presented as the mean±SD of four independent experiments *p<0.05, ** p≤ 0.01.
|
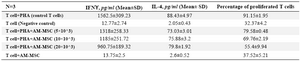
Table 1. IFNƳ, IL-4 and percentage of proliferated T cells after co-culture of T lymphocytes and hAM-MSCs (N=3)
|
|