Male Pronuclear Formation using Dog Sperm Derived from Ectopic Testicular Xenografts, Testis, and Epididymis
-
 Shirazi, Abolfazl
Reproductive Biotechnology Research Center, Avicenna Research Institute, ACECR, Tehran, Iran, Tel: +98 21 22404144; Email: a.shirazi@avicenna.ac.ir
Shirazi, Abolfazl
Reproductive Biotechnology Research Center, Avicenna Research Institute, ACECR, Tehran, Iran, Tel: +98 21 22404144; Email: a.shirazi@avicenna.ac.ir
-
Reproductive Biotechnology Research Center, Avicenna Research Institute, ACECR, , Tehran, Iran
-
Department of Gametes and Cloning, Research Institute of Animal Embryo Technology, Shahrekord University, Shahrekord, Iran
-
Khadivi, Asma
-
Department of Gametes and Cloning, Research Institute of Animal Embryo Technology, Shahrekord University, Shahrekord, Iran
-
Shams-Esfandabadi, Naser
-
Department of Gametes and Cloning, Research Institute of Animal Embryo Technology, Shahrekord University, Shahrekord, Iran
Abstract: Background: Testis tissue xenografting and the resultant sperm in a xenograft may provide a unique approach to rescue the genetic material of males that die prematurely and is a model for the study of human spermatogenesis and can represent an alternative approach for fertility preservation in cancer patients. This study was aimed to evaluate the xenogenic dog sperm in formation of male pronucleus following injection into the sheep oocytes.
Methods: The in vitro matured slaughterhouse derived sheep oocytes were subjected to Intracytoplasmic Sperm Injection (ICSI) with epididymal, testicular, and xenogenic dog sperm. The ICSI was performed after scoring of the sperm midpiece using an IX71-Olympus inverted microscope with Nomarsky optics. Within 1 hr after injection, the injected oocytes in activated group were exposed to 5 µM ionomycin for 5 min. The data were analyzed by Chi-square and ANOVA using SigmaStat, version 3.5, and p<0.05 was considered significant.
Results: The formation of female pronucleus after ICSI of xenogenic sperm was higher than epididymal and testicular sperm in non-activated oocytes. The corresponding rate in activated oocytes was higher or comparable with testicular and epididymal sperm. The rate of male pronucleus formation after ICSI of xenogenic sperm was comparable with injection of two other sperm sources. Oocyte activation had an inductive role in female and male pronuclear formation.
Conclusion: Dog xenogenic sperm was capable to induce oocyte activation and proportion of male pronucleous formation was comparable to the testicular and epididymal sperm.
Introduction :
Xenografting of testis tissue, from diverse species, in recipient immunodeficient mice has provided a tool for the study and restoration of the male germ line 1 and it paved the way for fertility preservation. Collection of sperm from males among valuable species that die before reaching sexual maturity and rare animal breeds or lines is another application of this technique. Additionally, the resultant xenogeneic sperm could be an alternative option for patients recovered from cancer to circumvent the risk of re-introduction of neoplastic cells as compared to auto-transplantation of isolated
germ cells 2.
Complete spermatogenesis including sperm production from immature donor mice, hamsters, cats, rabbits, pigs, goats, sheep, cattle and rhesus monkeys was successfully performed using the xenografting technique 1, 3-7.
Generally, when using the tissues of neonatal donor animals, the xenogeneic sperms are fertilization-competent after ICSI 1 and support the development of embryos 1,4,8 and birth of offspring 9.
The ICSI in animal models is a powerful approach that can be used for studies related to species-specific fertilization processes and to assisted human reproduction. Interspecies ICSI has been carried out to understand species-specific differences in oocyte environments and sperm components during fertilization. For instance, human sperm injection into mouse and rabbit oocytes has been used widely for studies related to assisted human reproduction 10,11.
In this context, the male chromatin decondensation could be used as a predictive test for fertilization capability of both sperm and oocyte after ICSI. The structural or biochemical defects in chromatin packaging during spermatogenesis are associated with failure in sperm decondensation in the oocytes 12. However, the complete decondensation of the human sperm nucleus after ICSI was reported as oocyte activation-dependent 13. Therefore, it could be concluded that sperm chromatin decondensation may be a predictive index for assessment of the fertilization potential of spermatozoa in the ICSI program.
There are evidences indicating the difference in motility, morphology, degree of maturity, chromosomal or DNA composition, and gene imprinting conditions between sperms from different sources 14-19. In the current study, the ability of male pronuclear formation in xenograft derived canine sperm after ICSI was compared with testicular and epididymal sperms. However, due to the low availability of biological material (bitch ovaries) and the inefficient developmental competence of canine oocytes matured in vitro, the sheep oocyte, for the first time, was employed.
Materials and Methods :
Except where otherwise indicated all chemicals were obtained from the Sigma (St. Louis, MO, USA).
Oocyte collection: The slaughterhouse sheep ovaries were collected and transported to the laboratory in saline (30-35ºC) in a thermos flask within 1-3 hr following collection. Ovaries were washed three times with pre-warmed fresh saline (37ºC), and all visible follicles with a diameter of 2-6 mm were aspirated using gentle vacuum (30 mmHg) via a 20 gauge short beveled needle connected to a vacuum pump. Prior to aspiration, the collecting tube was filled with 2 ml preincubated hepes-modified TCM, supplemented with 50 IU/ml heparin.
In vitro maturation of recipient oocyte: After aspiration, only oocytes surrounded by more than three layers of unexpanded cumulus cells (COCs: Cumulus Oocyte Complexes) were recovered and selected for In Vitro Maturation (IVM). The Oocyte Culture Medium (OCM) consisted of bicarbonate-buffered TCM 199 with 2 mM L-glutamine supplemented with 0.02 mg/ml cysteamine, 1 IU/ml hCG, 0.05% IU⁄ml FSH, 100 IU/ml penicillin, 100 mg/ml streptomycin, 10% FBS (fetal bovine serum, Gibco 10270), and 0.2 mM Na-pyruvate. The medium osmolarity was adjusted to 275 mOsm. The COCs were randomly distributed in maturation droplets (10 oocytes in 50 ml) and covered by sterile paraffin oil in a 60 mm Petri dish (Falcon 1008; Becton & Dickinson, Lincoln Park, NJ) and were then incubated under an atmosphere of 5% CO2 and 95% air with 100% humidity at 39ºC for 24 hr.
Source and preparation of spermatozoa: The xenogenic sperm was prepared in biomedical department, university of Saskatchewan 20. Briefly, the testis tissue from 2-mo-old donor dogs (Border-Collie cross puppies) was grafted on the back skin of recipient immunodeficient nude castrated male mice (NCr, nu/nu; Taconic, Germantown, NY, USA) at approximately 6 weeks of age. The recipient mice were sacrificed at 13 months postgrafting. The recovered xenografts were dispersed using fine forceps and 26-gauge needles attached to 1 ml syringes and then cryopreserved until use. In addition to xenograft, spermatozoa were also obtained from the epididymis and testis of mature dogs (mixed Iranian breeds) for comparison. Briefly, after incision on cauda-epididymis and testicular tissue, the collected fluids were diluted in base medium (tris+citric acid+glucose) and after centrifugation (700 g, 8 min), the collected samples were then cryopreserved in two steps with freezing medium composed of 3% and 7% glycerol and 20% egg yolk based on Neagu et al’s protocol.
The cryopreserved samples were then thawed and centrifuged for 10 min at 600 g and the supernatant was discarded. Washing was repeated 3 times and the pellet was resuspended in injection medium (Hepes bufferd Synthetic Oviductal Fluid: HSOF) and maintained at room temperature until use for ICSI.
Intracytoplasmic sperm injection: ICSI was performed using an IX71-Olympus inverted microscope with Nomarski optics (IX71Olympus, Tokyo, Japan). After IVM, the oocytes were denuded and in a group of 15 oocytes were placed into a drop of 50 ml of injection medium (HSOF) covered with mineral oil. The prepared sperm was diluted 1:1 with 12% polyvinylpyrrolidone in PBS immediately, just before microinjection. One droplet of injection medium (50 µl) with two droplets (10 µl) of PVP diluted sperm was arranged in two columns on the lid of a 60 mm tissue culture dish (Falcon 1008; Becton & Dickinson, Lincoln Park, NJ, USA). The injection and holding pipette had an inner diameter of 6 mm and 20-30 mm, respectively. The injection of a spermatozoon into an oocyte’s cytoplasm was performed using the method described by Shirazi et al. Briefly, sperms after the scoring of the midpiece were individually aspirated tail-first into the injection needle and injected into the ooplasm through the zona pellucida. The first polar body was either at 6 or 12 o’clock position, and the injection pipette was at 3 o’clock position. During the injection, cytoplasm was aspirated to approve that the oolema was broken. The spermatozoon was injected into the ooplasm with a minimum volume of medium (<5 pL) at 9 o’clock position. After ICSI, oocytes were washed three times in HSOF containing 6 mg/ml BSA and then randomly subjected into the activation and non-activation groups.
Activation of ICSI ova: Within 1 hr after injection, the injected oocytes (ICSI and Sham groups) were activated by exposure to5 µM ionomycin in H-SOF with 1% FBS for 5 min. Thereafter, the oocytes were washed in HSOF and placed into 20 µl drops of IVC medium.
Assessment of prunuclei formation: Male and female pronuclei formation was evaluated after staining with Hoechst 16 hr after ICSI. Oocytes with one pronucleus and a non-decondensed sperm head were considered activated, and those with two pronuclei and without a non-decondensed sperm head were considered fertilized.
Statistical analysis: Data were collected with at least three replicates. All proportional data were subjected to an arc-sine transformation, and the transformed values were analyzed using one-way ANOVA. When ANOVA revealed a significant difference, the treatments were compared by Fisher LSD method. Chi-square and Fisher Exact Test were applied for qualitative evaluation. All analyses were conducted with SigmaStat, version 3.5 and p<0.05 was considered significant.
Results :
Among three different sources of sperm, it was the testicular sperm that its decondensation after interspecies ICSI (Figure 1) was significantly (p<0.05) influenced by oocyte activation followed by ICSI as compared with non-activated ones (38±10 vs. 2.1±2.1). The corresponding percentages in epididymal and xenograft samples in activated oocytes were higher and lower respectively, though insignificant, as compared with non-activated oocytes.
In this experimental condition, the majority of injected oocytes failed to form the male pronucleus and had intact, decondensed or swelled sperm heads (Figures 2A-C). The majority of injected oocytes, however, were completely activated because they were released from MII arrest and had formed the female pronucleus (Figures 2A-C, E, F; Table 1). The female pronucleus was significantly higher in activated oocytes as compared to non-activated ones in all 3 different sources of sperm. In non-activated oocytes, the highest female pronuclear formation rate (p<0.05) was observed in those injected with xenograft sperm (Table 1). The proportion of oocytes with 2 pronuclei and without sperm head, activated oocytes with female and male pronuclei, were higher in activated oocytes (Figure 2E; Table 1). The difference between activated and non-activated oocytes, however, was significantly different only in those injected with epididymal sperm (Table 1). Proportions of both the Metaphase Plate (MP) and condensed forms of nucleus in non-activated oocytes were higher than activated oocytes (Figure 2D).
Discussion :
Testicular tissue grafting into immunodeficient mice has become an interesting and promising scientific tool for the generation of gametes and the study of testicular function. Complete spermatogenesis including sperm production from immature donor mice, hamsters, cats, rabbits, pigs, goats, cattle or rhesus monkeys was successfully performed using the xenografting technique 1,3-7. Moreover, with advent of ICSI, using this approach as a heterologous ICSI could represent a powerful tool as a predictive test for fertilization capability and to study sperm functionality especially in those species with scarce biological materials and low availability of mature oocytes (e.g. canine or wide species).
As shown, the xenogenic sperm head after ICSI into the sheep oocyte was decondensed as such in non-activated oocytes the corresponding rate was even higher than testicular sperm, though insignificant. The proportion of decondensed sperm head, however, in oocyte injected with xenogenic sperm following activation was significantly lower than epididymal sperm. Regarding the effect of post ICSI oocyte activation, the activation of oocytes could significantly increase the rate of decondensed sperm head in testicular sperm (2.1±2.1 vs. 38±10). These results suggest that xenogenic sperm head is capable of being decondeced like epididymal and testicular sperm and that the activation treatment may improve male chromatin decondensation based on sperm source. Moreover, considering the point that damaged sperm DNA may contribute to the failure of sperm decondensation after ICSI, it seems the xenogenic sperm might have an intact DNA like testicular and epididymal sperm 23.
The formation of female and male pronuclei was promoted by oocyte activation following ICSI. The difference in percentages of female pronucleus formation between activated and non-activated oocytes in all experimental groups were significant. Interestingly, the corresponding rate in oocytes injected with xenogenic sperm was higher than those injected with testicular and epididymal sperm (Table 1). In other words, in xenograft group, the proportion of female pronuclear formation in non-activated oocytes was significantly higher than (p<0.05) the corresponding rates in other groups. Similarly, in activated oocytes, the correspon-ding rate in xengraft group was higher than the rate in activated epididymal group (Table 1). From above, it could be concluded that the mechanisms involved in sperm-induced oocyte activation in xenogenic sperm are quite working.
With respect to the male pronucleus formation, activation of oocytes following ICSI could improve the development of male pronucleus formation in activated oocytes as compared to non-activated ones as such the difference has become significant in epididymal group. The association between sperm head decondensation and oocyte activation was confirmed by previous studies 13,24-27. Similarly, significant enhancement to the degree of transformation of the sperm nucleus into the Male Pronucleus (MPN) after ICSI was shown by oocyte activation 26,27.
Additionally, there was a report indicating the increase in the rate of recondensation of sperm heads when the injected oocytes were not stimulated 28. Therefore, higher rates of metaphase plate and condensed chromatin in non-activated oocytes as compared to the activated ones in this study (Table 1) might be related to the lack of artificial induction of oocyte stimulation following ICSI in the former.
Considering the verification of fertility competence of sperm derived from testis tissue xenografts by the generation of blastocysts and also offspring in pig, monkey, mouse, and rabbit using ICSI 4,7,8 and also the results of the current study, it seems xenografting of dog testes can be a promising scientific tool for the generation of male gamete and fertility preservation in this species.
Conclusion :
In conclusion, the resultant dog xenogeneic sperm is capable of being decondensed and to induce oocyte activation and finally transform to the male pronucleus after heterologous ICSI in sheep oocytes.
Acknowledgement :
The authors would like to thank the Research Institute of Animal Embryo Technology for technical and financial supports, Shahrekord University, Dr. Honaramooz and Dr. Abbasi for preparation of the xenograft, University of Saskatchewan.
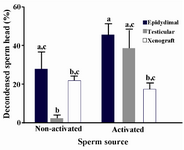
Figure 1. The oocyte activation and the proportion of decondensed dog sperm head with different sources after intracytoplasmic sperm injection into the sheep oocyte a-c The column with different superscript letter differs significantly (p<0.05)
|
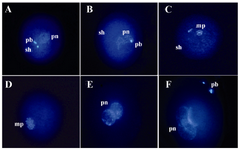
Figure 2. Nuclear morphology of sperm head after injection into the MII stage sheep oocytes stained with Hoechst, 16 hr after ICSI: A) An oocyte with two polar bodies (pb), one female pronucleus (pn), and one sperm head (sh) suggesting oocyte activation after ICSI; B) Oocyte with swollen sperm head; C) Oocyte with metaphase plate (mp); D) Oocyte with two metaphase plates; E) Oocytes with two pronuclei and without sperm head suggesting normal fertilization; F) Oocyte with three pronuclei suggesting abnormal fertilization
|
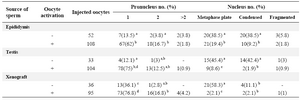
Table 1. Dog sperm head decondensation with different sources after intracytoplasmic injection into sheep oocytes
a-d) Numbers with different lowercase superscript in the same column differ significantly (p<0.05)
|
|