Evaluation of the Effect of miR-26b Up-Regulation on Hb-F Expression in Erythroleukemic K-562 Cell Line
-
Alijani, Sadegh
-
Department of Hematology, Allied Medical School, Tehran University of Medical Sciences, Tehran, Iran
-
 Alizadeh, Shaban
Department of Hematology, Allied Medical School, Tehran University of Medical Sciences, Tehran, Iran , Tel: +98 21 86704617 ;Email: alizadehs@sina.tums.ac.ir
Alizadeh, Shaban
Department of Hematology, Allied Medical School, Tehran University of Medical Sciences, Tehran, Iran , Tel: +98 21 86704617 ;Email: alizadehs@sina.tums.ac.ir
-
Department of Hematology, Allied Medical School, Tehran University of Medical Sciences, Tehran, Iran
-
Kazemi, Ahmad
-
Department of Hematology, Allied Medical School, Tehran University of Medical Sciences, Tehran, Iran
-
Kashani Khatib, Zahra
-
Students Scientific Research Center (SSRC), Allied Medical School, Tehran University of Medical Sciences, Tehran, Iran
-
Soleimani, Masoud
-
Department of Hematology, Tarbiat Modares University, Tehran, Iran
-
Rezvani, Mohamadreza
-
Department of Hematology, Allied Medical School, Tehran University of Medical Sciences, Tehran, Iran
-
Minayi, Neda
-
Department of Hematology, Allied Medical School, Tehran University of Medical Sciences, Tehran, Iran
-
Karami, Farshid
-
Department of Hematology, Allied Medical School, Tehran University of Medical Sciences, Tehran, Iran
-
Tayebi, Behnoosh
-
Department of Hematology, Qaem Hospital, Mashhad University of Medical Sciences, Mashhad, Iran
Abstract: Background: The major hemoglobin in the fetus is hemoglobin F (𝛼2𝛾2), whereas in adult humans, hemoglobin A (𝛼2𝛽2) is predominately expressed. Several studies have indicated that expression of the HbF subunit 𝛾-globin might be regulated post-transcriptionally. This could be done by small non-coding RNAs called microRNAs which target mRNAs in a sequence-specific manner and lead to translational repression or mRNA decay. The aim of this study is to evaluate the effect of miR-26b up-regulation on 𝛾-globin gene expression in K-562 cell line.
Methods: These cells were grown in RPMI 1640 and pre miR-26b and were transfected within K-562 cell line using lentiviral vector. After RNA extraction and cDNA synthesis in selected days, miRNA up-regulation was confirmed by miRNA real time PCR and then 𝛾and 𝛽chain and GATA-1 expression were investigated by RT and QRT-PCR.
Results: The viability of cells before transfection was 90%. Three and 7 days after transfection, through the use of relative Q-PCR, the 𝛾 chain expression increased 3.7, 6.8 and 3.8 folds and GATA-1 expression increased 2.1, 6.0 and 8.0 in comparison with untransfected cells.
Conclusion: The data suggest that miR-26b can be involved in the increase of 𝛾-globin gene expression in K-562 cell line. We suggest that miR-26b may be a significant therapeutic target for increasing HbF levels in patients with sickle cell disease and 𝛽-thalassemia.
Introduction :
Fetal hemoglobin, HbF (𝛼2𝛾2), is the major hemoglobin synthesized up to birth, but it decreases afterwards and adult hemoglobin, HbA (𝛼2𝛽2), becomes predominant 1,2. 𝛽-hemoglobin disorders, sickle cell disease and
𝛽-thalassemia are the main causes of morbidity and mortality worldwide. In these disorders, it is known that elevated HbF level is beneficial and as a compensatory mechanism causes significant decrease in clinical complications. Several studies showed that hemoglobin F expression might be post-transcriptionally regulated 3-5.
MicroRNAs (miRNAs) are 18-24 nucleotide (nt) small and are considered as noncoding RNAs that target cognate mRNAs at a posttranscriptional level by degradation or at a translational level by repression through base pairing 6-8. Many studies have focused on the significance of miRNA expression for HbF induction. In 2011, Walker et al, investigated miR profiles in patients with sickle cell anemia who were treated with hydroxyurea (as a known hemoglobin F inducers). They found miR-26b was directly associated with increased HbF levels in these patients 9. The aim of this study is to assess the effect of up-regulation of miR-26b on HbF expression by analyzing expression of 𝛾 and 𝛽-globin gene mRNA and miR-26b expression in different days in K-562 cell line.
Materials and Methods :
K-562 cell line was cultured at 37C under a humidified atmosphere consisting of 95% air and 5% CO2 in RPMI 1640 medium (Gibco. USA), 10% Fetal Bovine Serum (FBS) (Gibco. USA), 50 units/ml penicillin and 50 /ml streptomycin.
The DNA extraction was performed on normal human blood, using DNA extraction kit (VioGene) and the extraction of plasmid DNA from pCDH (511-b1) vector was done by using plasmid DNA extraction kit (VioGene). PCR product and plasmid DNA were digested with restriction enzymes, XbaI and BamHI (Fermentas). After digestion, ligation of insert fragment and vector were conducted with T4 DNA ligase enzyme (Fermentas).
PCDH (511-b1) containing the insert fragment was transformed into the competent bacteria (STBL4) and cultured overnight on LB-agar. After 24 hr, single random clones were chosen, then cultured and plasmid DNA was extracted. The single positive clones, pCDH containing pre-miR-26b, were confirmed by colony PCR with primary primers, sequencing and digestion with XbaI and BamHI.
Lentiviral vectors expressing transgene were produced by transfecting a three plasmid system into producer cells. Packaging plasmid ps-PAX2, envelope plasmid pMD2.G and pCDH-CMV-MCS-EF1-copGFP vector plasmids (with insert fragments) were transfected into HEK-293 cells, using the calcium–phosphate method.
𝛾-globin mRNA and 𝛽-globin mRNA as well as control GAPDH mRNA were quantified using SYBER GREEN master mix (Bioer) with Bioer’s thermal cycler according to manufacturer's protocol (94ºC 2 min, 94ºC 10 s, 58ºC 15 s, 72ºC 25 s) in 40 cycles. For single miRNA analysis, RNA isolated from K562 cell culture was used for real time PCR quantification using the high-specificity miRNA QPCR Core Reagent Kit (Stratagene, USA).
Results :
miR-26b was successfully amplified from human genome by PCR and a 380 bp product was obtained. Plasmid pCDH (511-b1) was extracted and this product was electrophoresed on 1% agarose. After transformation and overnight culturing of pCDH on LB-agar medium, single positive clones were selected and were successfully amplified by primary primers. Cells in log phase were selected for miR-26b transfection. Transfection was done in duplicate; since pCDH vector has GFP marker, K-562 cells that were infected by viral particles emitted shiny green color in inverted fluorescence microscope.
miRNA 26b up-regulation confirmation: miRNA real time PCR was performed on miRNA-specific cDNA synthesized from samples on third, seventh and fourteenth days after transfection.
Analysis of results showed 6, 11 and 24 fold increase in the expression of miR-26b on days 3, 7 and 14 in comparison to control cells (untransfected cells) on the same days (Figure 1).
Gamma, beta and GATA-1 expression through real-time PCR-based quantification: By relative Q-PCR, the expressions of chains were compared on three different days after starting transfection:
On the third day after transfection, the gamma (), beta () and GATA-1 expression were increased 3.7, 0.3 and 2.1 fold, respectively (Figure 2).
On the seventh day after transfection, the gamma, beta and GATA-1 expression were increased 6.8, 0.33 and 6.0 fold, respectively (Figure 2).
On the fourteenth day after transfection, the gamma, beta and GATA-1 were increased 3.8, 1.1 and 8 fold, respectively (Figure 2).
All chain expressions were evaluated in comparison with untransfected cells (control cells).
Discussion :
In this study, we investigated the effect of miR-26b up-regulation on HbF expression without any drug or any treatment by 𝛾-inducers. Initially, we assessed pre miR-26b expression in both groups of case and control on days 3, 7 and also 14 after the beginning of transfection. In our study, we assessed the expression of beta chain but we did not find any significant expression in this hemoglobin chain in comparison to the control group (p>0.05). We also compared the expression of beta chain on days 3, 7 and 14 and we did not find and statistically significant changes (p=0.2, p=0.4).
In fetal erythropoiesis, GATA-1 expression is highly up-regulated in terminal stages of erythropoiesis 10. We found that GATA-1 expression increased 2.1 fold on day three and this over expression was statistically significant in comparison to the control group (p<0.05). Comparison of GATA-1 expression on three different times also revealed statistically significant difference (p<0.05).
miRNAs have repressive features evident from the role GATA-1 plays on induction of HbF in terminal stages of erythropoiesis in fetus 10. Therefore, GATA-1 was highly over expressed by miR-26b up-regulation in our study. So GATA-1 does not seem to be a direct miR-26b target gene. The exact mechanism of GATA-1 over expression by miR-26b is not clarified in our study.
In our study, over expression of gamma chain was observed on days 3 and 7. On both days, the difference in number of case cells with controls was statistically significant (p<0.05). Statistical analysis showed that comparison of these two times revealed significant differences (p<0.05). But expression of gamma chain was suppressed on day 14. It seems that the effect of miR-26b on K562 cell was diminished on the third time but even this time, the difference between case cells and controls was statistically significant (p<0.05).
In the study of Aisha Walker et al, the miR profiles were assessed in patients with sickle cell anemia. They evaluated miR profiles in these patients before and after the administration of hydroxyurea and found that miR-151-3p and miR 26b were increased after the administration of drug. In their patients, miR-26b and miR-151 increase was accompanied by hemoglobin F induction by hydroxyurea 9.
Conclusion :
In conclusion, we found that miR-26b had an indirect positive effect on gamma chain expression in K562 cells. But further studies are needed to evaluate whether miRNA regulation also interferes with the hemoglobin switch occurring during pre-and postnatal development.
Acknowledgement :
This study was approved and supported by Tehran University of Medical Sciences (Project number: 91-01-31-16945).
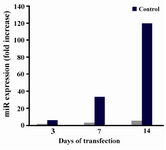
Figure 1. miR-26b expression on three different days of start-ing transfection
|

Figure 2. Effect of miR-26b up-regulation on erythroid dif-ferentiation of K-562 cells. The miR-26b was transfected into K-562 cells. The cells were assayed for gene expression of A) 𝛾, B) 𝛽 chains and C) GATA-1 on days 3, 7 and 14 after transfection in comparison to control cells (untransfected cells). Expression of these chains was detected by qRT-PCR. Relative fold changes of gene expression were calculated by ∆∆Ct method and the values were expressed as 2Λ-ΔΔCt
|
|