Assessment of Different Permeabilization Methods of Minimizing Damage to the Adherent Cells for Detection of Intracellular RNA by Flow Cytometry
-
Amidzadeh, Zahra
-
Diagnostic Laboratory Sciences and Technology Research Center, Shiraz University of Medical Sciences, Shiraz, Iran
-
Student Research Committee, Shiraz University of Medical Sciences, Shiraz, Iran
-
 Behzad-Behbahani, Abbas
Diagnostic Laboratory Sciences and Technology Research Center, Shiraz University of Medical Sciences, Shiraz, Iran, Tel: +98 711 2270301; Email: Behzadba@gmail.com
Behzad-Behbahani, Abbas
Diagnostic Laboratory Sciences and Technology Research Center, Shiraz University of Medical Sciences, Shiraz, Iran, Tel: +98 711 2270301; Email: Behzadba@gmail.com
-
Diagnostic Laboratory Sciences and Technology Research Center, Shiraz University of Medical Sciences, Shiraz, Iran
-
Erfani, Nasrollah
-
Shiraz Institute for Cancer Research, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran
-
Sharifzadeh, Sedigheh
-
Diagnostic Laboratory Sciences and Technology Research Center, Shiraz University of Medical Sciences, Shiraz, Iran
-
Ranjbaran, Reza
-
Diagnostic Laboratory Sciences and Technology Research Center, Shiraz University of Medical Sciences, Shiraz, Iran
-
Moezzi, Leili
-
Diagnostic Laboratory Sciences and Technology Research Center, Shiraz University of Medical Sciences, Shiraz, Iran
-
Aboualizadeh, Farzaneh
-
Diagnostic Laboratory Sciences and Technology Research Center, Shiraz University of Medical Sciences, Shiraz, Iran
-
Okhovat, Mohammad Ali
-
Diagnostic Laboratory Sciences and Technology Research Center, Shiraz University of Medical Sciences, Shiraz, Iran
-
Alavi, Parniyan
-
Diagnostic Laboratory Sciences and Technology Research Center, Shiraz University of Medical Sciences, Shiraz, Iran
-
Azarpira, Negar
-
Shiraz Transplant Research Center, Shiraz University of Medical Sciences, Shiraz, Iran
Abstract: Background: Various fixation and permeabilization techniques have been developed for detection of intracellular antigens by flow cytometry; however, there are few studies using flow cytometry to detect the frequency of intracellular nucleic acids, particularly RNA. We tested six different permeabilization methods in order to gain access to a high quality method with minimal damage to intracellular components focusing on 18S rRNA in HeLa cells.
Methods: HeLa cells were fixed in 2% paraformaldehyde. A variety of detergents and enzymes including saponin, TritonX-100, Tween-20, NP40, Proteinase K, and streptolysin O were used to optimize a protocol of permeabilization for the flow cytometric enumeration of intracellular 18S rRNA. Treated cells were subjected to standard protocol of flow cytometric in situ hybridization in the presence of FITC-labeled sense and antisense probes to detect 18S ribosomal RNAs. Samples were then analyzed on a FACSCalibur flow cytometer. To evaluate cell morphology, following hybridization the cells were fixed on glass slide, covered with DAPI, and evaluated on a fluorescent microscope with appropriate filter sets.
Results: In comparison with other methods, maximum cell frequency in percentage and fluorescent intensity (M1=2.1%, M2=97.9%) were obtained when the cells were treated with 0.2% Tween-20 and incubated for 30 min (p=0.001).
Conclusion: Our study indicated that the highest levels of mean fluorescence could be obtained when the cells were treated with Tween-20. However, it should be taken into consideration that for a successful flow cytometric result, other interfering factors such as hybridization conditions should also be optimized.
Introduction :
The development of sensitive detection methods such as flow cytometry techniques has allowed identification and enumeration of intracellular particles such as proteins and nucleic acids 1,2. So far, however, there are few studies using flow cytometry to detect and count intracellular nucleic acids, particularly RNA 3,4. The application of this fluorescent RNA detection method has a potential role not only in clinical diagnosis of infectious diseases such as viral infections, but also in opening a door for prediction and prognosis of cancer. For any flow cytometry application, cells must be prepared so that they are single and suspended in an appropriate medium. There are some factors influencing the choice of cell preparation procedures 5,6. If cell function would not be the case for investigation, there can be some advantages to fixing the cells for many other studies.
There are a variety of procedures for preparing cells, and the applied method will depend upon the cell type being investigated 7-9. However, selection of cell fixation and cell permeabilization method is crucial to achieve the best possible results and it leaves cell morphological scatter characteristic intact 10.
If internal nucleic acids are to be detected by labeled probes, the plasma membranes must be permeabilized prior to or during staining so that the nucleic acid probes are allowed to enter the cell. At the same time, the nucleic acid must be retained in the cell and morphology and the light scattering characteristics of the cell must be preserved. Generally, cells are fixed with paraformaldehyde, which stabilizes the membrane and increases its permeability, and the cells are further permeabilized with another agent 11.
Although fixation helps to preserve the cell structure and natural biological state of the sample, access to intracellular antigen or nucleic acids (DNA or RNA) in the cell requires permeabilization step. No single fixation/permeabilization method has been shown to be suitable for every cell line or antigen. Therefore, it is necessary to choose the optimal condition for each sample or cell line empirically. To find a proper method for intracellular identification of a particular antigen or nucleic acid, one needs to optimize factors including fixative/detergent concentration, exposure time, and temperature.
In this study, to determine the best permeabilization method, we investigated the effect of several permeabilization methods on the Hela cell line, as an adherent type of cell. Protecting intracellular components from damage and emphasis on nucleic acids (RNA in particular) were the main considerations of the study. A major type of cancer, carcinomas, is of epithelial cell origin. Since the HeLa cell line is a type of carcinoma cell, our suggested method would be applicable for intracellular detection of RNA especially mRNA by flow cytometry in cancerous tissues.
Materials and Methods :
Cell line: The HeLa cell line was originally obtained from ATCC (USA) and incubated in culture flasks in RPMI-1640 supplemented with 10% fetal calf serum, 100 g/ml penicillin and 100 U/ml streptomycin (Sigma, USA). Cell cultures were grown at 37°C with 5% CO2. Cells were passaged and then detached and collected via enzymatic digest with 0.1% Trypsin-EDTA (Gibco, USA). Cells were harvested by trypsinization and pooled with the medium. Cells were washed twice in PBS and pelleted at 500 g for 10 min at room temperature. Using trypan blue assay, cell count and viability analysis were performed so that the final cell concentration was 2×106cells/ml.
Fixation and permeabilization protocols: HeLa cells (2×106cells/ml) for flow cytometry were fixed in 2% cold and freshly prepared paraformaldehyde in Phosphate Buffered Saline (PBS). Samples were then incubated at room temperature for 15 min with slow shaking. Following cross-linking fixation, the plasma membrane must be permeated to allow entry of cell-impermeable fluorescent probes. This may be achieved by treatment with enzymes or detergents. In this study, four different detergents and two different enzymes were used to achieve staining protocols.
Method I (Saponin): In order to remove extra-fixative reagents, HeLa cells were washed with 1X PBS and then centrifuged at 500 g for 5 min. For cell permeabilization, 200 l of saponin (Sigma, USA) at concentration of 0.1, 0.2 and 0.5% was added to each tube and incubated for 10, 20, and 30 min at 25C, respectively. The samples were then washed with 1×PBS to remove saponin from the medium. The cells were used for hybridization and flow cytometry analysis.
Method II (TritonX-100): The same procedure as method I (saponin) was performed with a little modification. In this method, the cells were incubated with TritonX-100 at concentrations of 0.1 and 0.2% each for 5 and 10 min at 25C.
Method III (Tween-20): The same procedure as method I (saponin) was performed. However, instead of saponin, the cells were incubated with Tween-20.
Method IV (NP40): The same procedure as method II (Triton X-100) was performed. In this method, instead of Triton-X100, the cells were incubated with NP40.
Method V (Proteinase K): In order to remove extra fixative-reagents, HeLa cells were washed with 1X PBS and then centrifuged at 500 g for 5 min. For cell permeabilization, 200 l proteinase K (Sigma) in concentrations of 0.01, 0.05 and 0.1 g/ml in Tris-HCl (20 mM) and CaCl2 (2 mM) were added to each tube and incubated for 5, 10, and 15 min at 37C. The samples were then washed with 1×PBS to remove proteinase from the medium. The cells were used for hybridization and flow cytometry analysis.
Method VI (Streptolysin O; SLO): Streptolysin O is readily oxidized in the solution. To be activated, SLO must be reduced by agents such as dithiothreitol (DTT). For this reason, SLO in concentrations of 0.2, 0.5 and 1 g/ml was incubated with 10 mM DTT for 30 min at 37C. HeLa cells were then added to the activated SLO mixture and incubated for 5-10 min on ice to allow the binding of SLO to the surface of the cells. The cells were then washed in 1×PBS, incubated at 37°C for 10 min to remove cell surface SLO from the medium and to allow cell permeabilization and then they were used for hybridization and flow cytometric analysis.
Oligonucleotide probe: In order to evaluate the efficacy of various permeability and hybridization methods, two 25-base-longs 5’- Fluorescein isothiocyanate (FITC)-labeled sense (as negative control) and antisense probes were designed to detect 18S ribosomal RNAs. The antisense sequence was: TCACCTCTAGCGGCGCAATACGA AT. The probe was used as a ubiquitous target transcript in HeLa cells.
In situ hybridization: Standard protocol of in situ hybridization was used to determine the best possible method of permeabilization for intracellular detection of RNA by flow cytometry. However, the distribution for incubation time and concentrations of different enzymes and detergents which were used in the methods were uneven. Briefly, after fixation and permeabilization (typically, each prepared cell contained 1×106 cells), the cells were washed with 1×PBS buffer and then spun down. Meanwhile, the appropriate amount of the probe prepared in hybridization buffer (2× SSC, 50% formamide, 1×Denhart’s solution, 50 mM NaH2PO4/Na2HPO4 and 100 µg/ml salmon sperm DNA, Dextran sulfate 10%) was heated at 80C for 3 min and cooled down on ice. The cells were suspended by repeated pipetting in 50 l of hybridization buffer containing 0.5 µg/ml of 18srRNA Probe. Hybridization was carried out at 40°C with gentle shaking overnight. After hybridization, 300 l of hybridization buffer without the probes was added to the cells and further incubated at 40°C for 45 min to remove mismatched probes. The cells were then pelleted and washed successively with 2×SSC and 0.1×SSC for 30 min each to remove non-specific binding. Finally, the cells were suspended in 1 ml 1×PBS buffer for flow cytometry experiments.
Flow cytometric analysis: Prepared samples were kept in the dark until analysis by flow cytometry. Samples were analyzed on a FACSCalibur flow cytometer (Becton Dickinson, USA) equipped with a 15 mW air cooled 488 nm argon-ion laser. Fluorescent (FITC) was detected using 530/30 filter. Standard polystyrene latex particles (2.54 ) were used for instrument calibration. The data were analyzed with cell quest software (BD, USA). A total of 10000 cells were acquired for each sample. Data were expressed as geometric mean fluorescence intensity and as ratio between the fluorescence emission of sample cells and that of the isotypic control (P/N ratio; positive/negative).
Statistical analysis: For comparison among the six permeabilization methods, the geometric fluorescence intensity mean of intracellular 18S rRNA was subjected to ANOVA analysis using SPSS software package (version 15).
Results :
Although in this study, cell fixation method was not compared with other methods, preservation of 18S rRNA fluorescence emission with standard protocol of 2% PFA for 15 min at room temperature had significant positive effect on Forward Scatter (FSC) and Side Scatter (SSC) results.
Comparison of different permeabilization methods: Figure 1 shows the mean percentages of the cells falling under the negative (M1) or positive (M2) parts of the flow cytometry histogram, as well as the geometric mean fluorescence intensity (GMFI) of the cells under M2 marker for 18S rRNA in HeLa cells. Referring to the results presented in figure 1, although all the six methods were capable of permeabilizing the cell population, clear-cut positivity was observed by using Tween-20 in comparison to other methods (p=0.001). The highest percentage of permeabilized cells (cells under M2 marker) was observed with Tween 20 and NP40 (94.1±2.3, 50.1±20). While Tween 20 significantly affected the Geometric Mean Fluorescent Intensity (GFMI) of the permeabilized cells (98.3±8.8.) NP40 had lower effect on the permeabilized cells in comparison to Tween 20 (48.62±12). The lowest GMFI was observed when TritonX-100 (43.8) was used. GMFI for streptolysin O, saponin and Proteinase K were 55.7±14, 61.7±19 and 82.6±17, respectively (Figure 1). Tween-20 permeabilization method caused a dramatic improvement in cell permeability with increasing quality in cell morphology and stability in environment.
Effect of detergent and enzyme concentrations on GMFI: In order to gain access to intracellular 18S rRNA and leave the morphological scatter characteristics of cells intact, HeLa cells in suspension were treated with different concentrations of detergents and enzymes in a range of incubation times. In preliminary tests with various detergents, Tween-20 gave the most promising results. Tween-20 at concentrations of 0.1, 0.2 and 0.5% was added to each tube containing 2x106cells/ml HeLa cells. Each tube was then incubated at 25C for 10, 20 and 30 min. Maximum cell frequency and fluorescent intensity (M1=2.1±2.3, M2=97.2±8.8) were obtained when the cells were treated with 0.2% Tween-20 and incubated for 30 min.
TritonX-100, on the other hand, had negative effect on fluorescent intensity when it was used at 0.2% concentration and incubated for 10 min (M1=98.3%±8.8, M2=1.7±2.3). The comparison results of all six permeabilization methods using nearly the same concentration for the average incubation time of 10 min is shown in figure 2. We investigated the concentration and the incubation time in which the highest fluorescent intensity was achieved. Accordingly, various concentrations of detergents and enzymes were tested to determine the maximum fluorescent intensity over different incubation time periods (Figure 3). Consequently, 0.2% Tween-20 for 30 min, 0.1% NP-40 for 10 min and 0.2% TritonX-100 for 5 min resulted in maximum fluorescent intensity. No obvious differences were observed in the shape of FSC/SSC dot plot after permeabilization with different permeabilization methods (Figure 4), suggesting no significantly different effects on the cell morphological scatter characteristic.
Discussion :
A major problem with the use of nucleic acid probes for detection of intracellular RNA is the cell membrane impermeability. Several strategies have been developed to solve the delivery problem 12. However, a simple strategy is to use a reliable cell permabilization technique in order to permit the oligonucleotide probe to gain access to the intracellular target. Thus far, there is no single fixation/permeabilization method which can provide the best result for every cell line; therefore, optimal conditions should be determined empirically.
The present study was implemented to optimize several protocols of permeabilization for the flow cytometric enumeration of intracellular 18S rRNA and promote consistency of the methodology used. FSC and SSC were recorded for a variety of different methods.
In method I, HeLa cells were fixed with 2% paraformaldehyde and permeabilized with saponin and the hybridization was performed in the presence of 5’-FITC- labeled antisense probe for intracellular detection of 18S rRNA. Saponin interacts with membrane cholesterol, selectively removes it and leases holes in the membrane. However, our preliminarily results suggested that no significant cell membrane alterations were caused by saponin. We attempted to improve the results by several modifications (e.g., saponin concentration and time of incubation), but no major improvement in MFI and M2 percentages (22-36%) was observed (p=0.13). We concluded that HeLa cell membranes were highly resistant to saponin permeabilization. Therefore, intracellular 18S rRNA would not be accessible to the probe. The same results were reported by Mercanti and Cosson as well 13.
TritonX-100 is one of the most widely used nonionic surfactants used to permeabilize the living cell membrane 14-18. In comparison with saponin permeabilization, up to 80% increase in fluorescence intensity was observed when cells were treated with 0.2% TritonX-100 for 5 min. However, a significant decrease in fluorescence intensity (1.87%) was observed when the cells were incubated for 10 min. Results indicated that TritonX-100 may act as a permeabilizing agent depending on the dose and duration of exposure to cells.
Tween-20 is a nonionic detergent and is able to solubilize cell membrane without affecting cell membrane integrity 19. It is able to create pores large enough for oligonucleotide probe to go through without dissolving the plasma membrane. It is suitable for antigens and nucleic acids in the cytoplasm.
Using Tween-20 in concentration of 0.2% for 30 min of incubation showed that the method was the best approach for cell permeabilization. Using method III, the fluorescence intensity of 18S rRNA was reached to the maximum amount among all the others (frequency of M2: 91.4-97.6%). Our results indicated that cell permeabilization with Tween-20 gave even higher MIF when compared with permeabilization with other methods. The reasons why MFI of intracellular 18S rRNA using Tween-20 is significantly higher than other permeabilization methods is unclear. However, chemical composition and impact of incubation time could account for the better performance of Tween-20 method.
Proteinase K is one of the most active endopeptidases known. Proteinase K rapidly inactivates endogenous nucleases including RNases and DNases 20. It creates pores in the cell membrane and facilitates access to DNA or RNA for labeling with in situ hybridization technique 21.
To obtain optimal reaction conditions, the exact enzyme concentrations and incubations must be determined empirically for different cells. However, in our experiment, no significant change in M2 (11.6-29.8%) was observed when different concentrations of the enzyme and incubation times were used (p0.05). Results indicated that direct use of proteinase K without additives such as SDS could not permeabilize the plasma membrane of the HeLa cells perfectly. In the presence of 0.1-0.5% SDS, proteinase K retains activity and will digest a variety of proteins and nucleases in DNA or RNA preparations without compromising the integrity of the isolated nucleic acid 22. Therefore, we suppose that oligonucleotide probe had incomplete access to the target in the cells.
Streptolysin O (SLO), prototype of the cholesterol-binding family of bacterial exotoxins, forms very large pores in the plasma membrane of mammalian cells 23. After binding to membranes, toxin monomers diffuse laterally in the bilayer and oligomerize to form homotypic aggregates that represent very large transmembrane pores. The correct SLO concentration must always be initially determined by titrations. In our study, HeLa cells treated with concentrations of 0.1 to 1 g/ml of SLO were incubated for 5 and 10 min. M2 frequency of 19.6-30.6% reflects that about 70% of the cells remain non-permeabilized. It could be explained that SLO has made the hole large enough for the probes to leave the cells. Therefore, hybridization did not occur.
Like Triton, Nonidet P-40 (NP-40), a nonionic, non-denaturing detergent can also partially dissolve the nuclear and plasma membrane of the cells. However, they are harsh detergents which can disrupt membrane proteins, especially if left for too long and are not suitable for membrane proteins. NP-40 was used for cell permeabilization in concentrations of 0.1 and 0.2% and for no longer than 10 min. In case of fluorescence intensity, the best result was obtained when the cells were treated with 0.1% NP-40 and incubated for 10 min (M2=71.2%). However, increasing NP-40 concentration to 0.2% resulted in a 20% decrease in fluorescence intensity (M2=50%). However, our results indicate that there is no significant difference between either using NP-40 or TritonX-100 for HeLa cell permeabilization.
Conclusion :
In conclusion, we have examined several methodological approaches that can be used to improve flow cytometric detection of intracellular 18S rRNA in HeLa cells. It is clear from our study that the highest levels of mean fluorescence could be obtained when the cells are treated with Tween-20. However, it should be taken into consideration that for a successful flow cytometry result, other interfering factors such as hybridization conditions should also be optimized. The method would be applicable for flow cytometry detection of intracellular mRNA in clinical samples with emphasis on viral infections and cancer.
Acknowledgement :
The authors are very thankful to Dr. N. Shokrpour for reviewing the manuscript. This work was financially supported by a grant no. 5992 from Shiraz University of Medical Sciences, Shiraz, Iran. The work was performed with the collaboration of Shiraz Institute for Cancer Research.
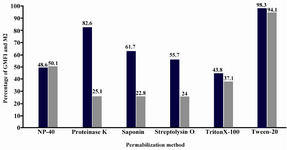
Figure 1. Flow cytometric analysis of 6 different permeabilization methods for detection of 18S rRNA in HeLa light color column: Geometric Mean Fluorescence Intensity (GMFI); Dark color column: Mean percentage of the cells in positive area (M2)
|

Figure 2. Flow cytometric histograms of 18S rRNA using the same concentration of detergents and enzymes activity during 10 min of incubation time
|

Figure 3. Maximum fluorescent intensity obtained by different permeabilization methods related to the detergents or enzymes concentrations and incubation time
|
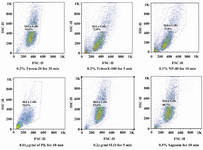
Figure 4. Comparison of forward and side scatter lights (FSC/SCC) of six permeabilization methods using different concentrations and incubation times. The FCS represents the size and SSC represents the internal granularity of the cells after permeabilization
|
|