Expression Enhancement in Trastuzumab Therapeutic Monoclonal Antibody Production using Genomic Amplification with Methotrexate
-
Akbarzadeh-Sharbaf, Soudabeh
-
Department of Industrial and Environmental Biotechnology, National Institute for Genetic Engineering and Biotechnology (NIGEB), Tehran, Iran
-
Yakhchali, Bagher
-
Department of Industrial and Environmental Biotechnology, National Institute for Genetic Engineering and Biotechnology (NIGEB, Tehran, Iran
-
Minuchehr, Zarrin
-
Department of Industrial and Environmental Biotechnology, National Institute for Genetic Engineering and Biotechnology (NIGEB), Tehran, Iran
-
 Zeinali, Sirous
Department of Molecular Medicine, Biotechnology Research Center, Pasteur Institute of Iran, Tehran, Iran, +98 21 88939140;zeinali@kawsar.ir
Zeinali, Sirous
Department of Molecular Medicine, Biotechnology Research Center, Pasteur Institute of Iran, Tehran, Iran, +98 21 88939140;zeinali@kawsar.ir
-
Department of Molecular Medicine, Biotechnology Research Center, Pasteur Institute of Iran, Tehran, Iran
-
Kawsar Human Genetics Research Center, Tehran, Iran
Abstract: Background: Trastuzumab (Herceptin) is a humanized monoclonal antibody (mAb) which is used for specific treatment of metastatic breast cancer in patients with overexpression of HER2/neu receptor. In this study, we have attempted to develop a biosimilar version of trastuzumab mAb.
Methods: According to in silico studies, the heavy and light chains of trastuzumab mAb were designed and constructed. The recombinant constructs were co-transfected in CHO DG44 cell line. Stable transformants were selected on a semi solid medium. Genomic amplification with methotrexate was achieved for heavy chain gene amplification. Biological activity of produced antibody in comparison with Herceptin was tested by flow cytometry method.
Results: Three folds of amplification were obtained after seven rounds of methotrexate treatments. The results indicated the equal expression level of heavy and light chains. The yield of purified mAb was between 50 to 60 mg/l/day. According to the results, the produced mAb had similar affinity to HER2+ tumor cells to that of Herceptin.
Conclusion: High-level recombinant protein expression can be achieved by amplification of the recombinant gene with a selectable marker, such as Dihydrofolate Reductase (DHFR). It is usually accepted that DHFR gene can be amplified in DHFR− CHO cells, which consequently leads to amplification of the co-linked target gene, and finally amplification of recombinant protein. In this research, with the aim of producing a biosimilar version of herceptin, the effect of genomic amplification was investigated on the increasing the gene copy number using quantitative real-time PCR.
Introduction :
Trastuzumab (Herceptin™) is the first commercially available mAb for treatment of Metastatic Breast Cancer (MBC) 1,2. It has been shown that it markedly inhibits the growth of HER2+ breast tumor cells. Clinical application of Trastuzumab was approved by the US Food and Drug Administration (FDA) in 1998 and also by the European Medicines Agency (EMA) in 2000, in women with HER2+ MBC to Genentech/Roche 3-5.
In 1986, Drebin et al, using hybridoma technology, were successful in isolating 4D5 murine monoclonal antibody (mumAb4D5) against the HER2/neu epidermal growth factor receptor. Hu4D5 (Trasuzumab) was initially created by Complementarity Determining Region (CDR) grafting loops obtained from the mumAb4D5 inserted into human IgG1 framework regions. However, tumor cell growth inhibition of the proto-type of hu4D5 was not detectable because of its low affinity to the antigen (~80-fold) compared to the murine 4D5. Affinity maturation by molecular modeling increased the affinity of this humanized antibody to three folds higher than that of the murine antibody. This maturation led to a significant tumor inhibition 6,7.
Tumor cell growth inhibition of Trastuzumab is not limited to metastatic tumors. Studies on tissues, in the early stage of malignancy, with overexpression of HER2/neu, indicated that it could prevent tumor emergence 8. Trastuzumab is now used as an adjuvant treatment for individuals with HER2+ breast cancer detectable in lymph nodes 9,10. In October 2010, the FDA granted approval for using of Trastuzumab in combination with cisplatin and fluoropyrimidine, for treatment of HER2+ metastatic gastric or Gastroesophageal (GE) patients who had not received any previous treatment.
Increasing demand for development of stable and high-producing cell lines for therapeutic protein production is a major concern in the biopharmaceutical industry 11,12. It is a common knowledge that in mammalian cells, gene expression is a complex process and comes under regulation at several different points, such as DNA modifications, transcription, translation, secretion, protein folding, etc. 13-15. Achieving a high producer and stable cell line, which express the protein with intact biological activity, is an essential part of producing a therapeutic recombinant protein.
Generation of stable cell lines producing recombinant antibody could be achieved with clonal selection. Scaling up high-producing clonal cells and genomic amplification with methotrexate (MTX) could be used to obtain a population of cells expressing high levels of recombinant antibody 16,17. The purpose of this study was to produce a biosimilar version of Trastuzumab therapeutic mAb using recombinant DNA technology. This study was performed using a DHFR deficient DG44 cell line derived from CHO cells 18.
Materials and Methods :
Construction of trastuzumab heavy and light chains expression vectors:The heavy and light chains (HC and LC respectively) of Trastuzumab therapeutics mAb (drug bank database ID: DB00072) were designed according to bioinformatics studies. Three-dimesional structure of this protein was obtained from the PDB: Worldwide Protein Data Bank (PDB ID: 1N8Z). The KV3A9-Mouse IgG kappa chain V-III (UniProt ID: P01661) was selected as secretory signal peptide. The sites of (http://slam.bs.jhmi. edu/gd/) and (http://www.kazusa.or.jp/codon/) were used for gene design and codon usage preference. RNA structure prediction was carried out with the use of genebee.msu.su/ services site.
The designed fragments were synthesized and cloned in pUC57 vector by Jarf Company (Tehran, Iran). The primer sequences were designed for amplification of HC: forward 5' TCTCGAGCACCATGGAG 3' and reverse 5' GAATTCTCATCACTTGCC 3' and for amplification of LC: forward 5' AGCTAGCCACCATGGAG 3' and reverse 5' CGTACGTCATCAGCACTC 3'. The appropriate sequences were amplified by Platinum® Taq DNA polymerase high fidelity kit (Invitrogen, USA). Two shuttle vectors (i.e. pcDNA™ 3.3-TOPO® and pOptiVEC™ TOPO®) (Invitrogen, USA) were used for final cloning. Restriction enzyme mapping (Fermentus, Lithuania) and sequencing (ABI Big Dye Terminator, ABI, USA) were performed for ten final selected constructs using recommended protocols.
Stable cell lines development:The CHO DG44 cell line (Invitrogen, USA) was initially co-transfected with pSLO (inserted LC in pOptiVEC™ TOPO® vector) and pSHC (inserted HC in pcDNA™ 3.3-TOPO® vector) constructs and then with pSLC (inserted LC in pcDNA™ 3.3-TOPO® vector) and pSHO (inserted HC in pOptiVEC™ TOPO® vector) constructs using FreeStyle™ MAX reagent and OptiPRO™ SFM (Invitrogen, USA). Stable transformants were selected using CD OptiCHO™ medium (Invitrogen, USA) containing 500 μg/ml Geneticin®. Clonal selection by limiting dilution in semi-solid media was performed with CloneMatrix semi-solid concentrated and CloneXL Reagent (Genetix, USA) in addition to CD OptiCHO™ cloning medium (2x, Invitrogen, USA). Plating was performed on 20 plates (96-well) with a density from 0.5 to 2 cells/well. The single-cell colonies were scaled up using 48, 24, 12 and 6-well plates and 25 and 75 cm3 flasks.
Genomic amplification by MTX selection: Seven rounds of genomic amplification by MTX selection were performed using amounts of MTX from 500 nM to 4 μM in complete CD OptiCHO medium. For each single cell clone, the cell seeding was performed in 6-well plates at densities of 2 to 5×105 cells/ml. Plates were incubated at 37oC under 6% CO2. During 2 or 3 weeks, the medium of each clonal cell line was changed with fresh medium containing MTX. When the cell viability reached 70%, the new round of selection was performed by increasing concentration of MTX. Antibody production was monitored during each round of amplification using SDS-PAGE method.
Genomic DNA (gDNA) extraction and multiplex PCR: The gDNA from each of the 18 stable transformants was extracted using the Wizard® Genomic DNA Purification kit (Promega, USA). Multiplex PCR was performed using Platinum® Taq DNA Polymerase High Fidelity kit (Invitrogen, USA) with available commercial primers for pOptiVEC™-TOPO® TA and pcDNA™3.3-TOPO® TA vectors. The forward primer sequence for amplification of the HC and LC was: 5' CGCAAATGGGCGGTAGGCGTG 3'. The reverse primer sequences for the amplification of the HC and LC were: 5' CCTTATTCCAAGCGGCTTCG 3' and 5΄ CTTCCGTGTTTCAGTTAGC 3΄ respectively. The PCR was performed by an initial denaturation step of 94ºC for 1 min; 30 cycles of 94ºC for 30 s, 55ºC for 30 s, 68ºC for 2 min, with a final extension of 68ºC for 20 min.
Protein purification: Affinity chromatography columns (HiTrap™ Protein A & Protein G HP, GE Healthcare, UK) were used for preparative purification of monoclonal IgG from cell culture supernatants. Each stable transformant was cultured in 125 ml flask containing 30 ml CD OptiCHO™ medium and 500 μg/ml Geneticin. When the cell density reached 107 viable cells/ml, the supernatant containing the secreted antibody was collected by centrifugation (6 min at 1000 g), concentrated and buffer exchanged with Centriprep 10 kDa (Millipore, USA). The monoclonal antibody was purified from the antibody-rich supernatant with HiTrap™ Protein A & Protein G HP columns. The protein concentration was determined using the UV absorbance at 280 nm with a Thermo Scientific NanoDrop 1000 spectrophotometer.
SDS-PAGE & western blot analysis: SDS-PAGE was carried out under reducing and non-reducing condition on resolving poly-acrylamide gel (10 and 12% w/v) according to Laemmli method 19. Western blot analysis was performed for determination of Trastuzumab antibody in stable transformants culture medium. For this purpose, when stably transfected CHO-DG44 cell lines were established, the culture supernatants were harvested and concentrated using an Amicon Ultra-15 filter with a 10 kDa cut-off. The concentrated supernatants were run on polyacrylamide gel and transferred to the PVDF membrane using a semi-dry blotting cell (Bio-Rad, USA). The HC and LC proteins were detected using goat anti-human IgG1 antibody conjugated with alkaline phosphatase (Sigma, Germany). Antigen-antibody complexes were visualized by BCIP/NBT solution (Sigma, Germany).
Quantitative real-time PCR: Quantitative real-time PCR assay was performed to estimate the number of HC gene copies inserted into the CHO DG44 cell line. Forward and reverse primers for amplification of the HC were 5' CCTACATCCACTGGGTGAGGC 3' and 5' CGGTGTTCTTGGAGGTGTCG 3' and for amplification of the LC were 5' AGGTGGAGATCAAGAGGACCGT 3' and 5' CCACCTTCCACTGCACCTTG 3' respectively. The housekeeping gene β-1,4-galactosyltransferase-1 was used as a reference control gene to normalize experimental results. For each experiment, an aliquot of SYBR green master mix (ABI, USA) and forward/reverse primers were added to each gDNA tube (i.e. from STD7, STD72G, STD76G and STD77G stable transformants) in a total volume of 25 µl. The reactions were carried out in duplicates with a StepOne real-time PCR system (Applied Biosystems, USA).
Flow cytometry analysis: Specific binding of the produced mAb to HER2/neu antigen was investigated using indirect flow cytometry method. The MDA-MB-361, MDA-MB-468, MCF7, SK-BR-3, MDA-MB-453, T-47D and SK-OV-3 cell lines were obtained from National Cell bank of Iran (NCBI, Pasteur Institute of Iran). All of these cell lines are isolated from human breast or ovarian malignant cells and could express the HER2 gene product in high, moderate or low levels.
Each cell line individually was cultured, harvested and washed with cold Phosphate Buffered Saline (PBS) and then analyzed by flow cytometry. The cells were treated with purified Trastuzumab antibody (1 μg/ml) as experiment, Herceptin antibody (1 μg/ml) as positive control and anti-human IgG1 (1 μg/ml) as isotype-matched control antibody. For each assay two additional controls were prepared; one with absence of any antibody and the other one without any primary antibody. The cells were stained with goat anti-human IgG FITC conjugate (1/10000) (Sigma, Germany) as the secondary antibody. All treated samples were analyzed with FACS Canto II (Becton Dickinson, USA) and the results were processed by FACS DIVA software (Becton Dickinson, USA).
Results :
Stable cell line development:The heavy and light chains of trastuzumab mAb were designed according to bioinformatics data, constructed in the pUC57 prokaryotic vector and amplified by PCR. For antibody expression, two PCR products (HC and LC) were cloned separately into pOptiVEC™ TOPO® TA and pcDNA™ 3.3-TOPO® TA vectors to create four expression vectors. Since antibody expression depends on the combination of vectors containing different subunits of the antibody, the transfection conditions were optimized using different combinations of constructs. Best expression results were obtained from the co-transfection of pSLC and pSHO constructs into CHO DG44 cell line.
Stable transformants were selected on a semi solid medium and 18 transformants, named STD 3-18, 20 and 21 were scaled up (Figure 1). Multiplex PCR showed that the HC and LC had been integrated into the genomic DNA for all transformants (Figure 2). In most transformants the LC protein was expressed at high level, but the yield for the HC was undetectable (Figure 3). Since the HC had been integrated into the CHO DG44 genome coupled with DHFR gene; genomic amplification using MTX was used for increasing the HC gene copy number. Only STD7 transformant showed acceptable response to this treatment. Seven rounds of genomic amplification were performed and four new transformants namely STD72G, 75G, 76G and 77G were obtained, all come from the STD7.
Clone stability studies were performed on these transformants in presence and absence of MTX selection. Culture medium was changed every 4 days and antibody secretion was investigated on the supernatant after every five passages. These studies were performed over 50 passages but no changes in bio-productivity, growth or viability were observed.
Quantitative real-time PCR: An assessment of HC gene copy number, in stable transformants after genomic amplification with MTX, was performed using quantitative Real-time PCR. Relative gene copy number was quantified by the comparative threshold cycle (∆∆CT) method 20. The gene index was calculated by subtracting the transgene assay threshold cycle from the control assay threshold cycle. In this assay the HC gene in the STD7 was used as reference sample and the LC gene in the STD7 was used as control. Analysis was done using stepOne software and the results of Delta-Delta Ct for the STD72G, 76G and 77G were obtained 0.58, 1.33 and 1.57, respectively. According to this data the STD72G, 76G and 77G showed 1.5, 2.5 and 3 fold increase in the HC gene copy number, respectively.
Protein expression analysis: SDS-PAGE and western blot analyses were performed to evaluate the expression profile of transfected cells on concentrated culture supernatant. Western blot analyses on the STD 3-18, 20 and 21 transformants indicated presence of a 25 kDa band related to the LC (Figure 3). SDS-PAGE analyses were carried out in reducing and non-reducing conditions for the STD7, STD72G, 76G and 77G transformants. A 25 kDa protein band of the LC and a 50 kDa for the HC were detected on 10% SDS-PAGE. In non-reducing condition, as shown in figure 4, the HC and whole Ab bands were seen in position 100 and 150 kDa respectively. Level of mAb production was also evaluated after 2, 6 and 7 rounds of genomic amplification (Figure 4). For confirming the complete secretion of produced antibody, the cell lysates from each clonal cell lines were extracted and analyzed by SDS-PAGE and Western blotting, but as we expected the antibody chains weren’t recognized in intracellular locations. Whereas the analysis of cell-free culture supernatants presented that the whole mAb efficiently secreted with the yields up to 50 mg/l/day.
Determination of biological activity: Binding specificity of the produced mAb to HER2/neu antigen was investigated using flow cytometry. Eight HER2+ cell lines, which express the HER2 membrane protein in low, moderate and high levels were selected and analyzed with indirect immunofluorescence staining. The cells were treated with purified Trastuzumab antibody as experiment, Herceptin antibody as positive control and anti-human IgG1 as isotype-matched control antibody. The flow cytometry results indicated that SK-OV-3 and SK-BR-3 cell lines (high HER2 receptor producer), showed higher binding affinity compared to MCF7, T-47D, MDA-MB-361 and MDA-MB-453 cell lines. MDA-MB-468 cell line had the least response. The results are illustrated in figure 5. Each positive sample showed a right shift in comparison with the isotype-matched control. Flow cytometry analysis of culture supernatants demonstrated that the produced mAb is fully functional after secretion and have similar affinity as Herceptin. These results also indicated the proper folding, correct assembly and biological activity of produced Trastuzumab antibody.
Discussion :
Mammalian cells, because of their capacity for proper protein folding, assembly, and post-translational modification are one of the most effective expression systems for recombinant protein production. High-level recombinant protein expression can be achieved by amplification of the recombinant gene with a selectable marker, such as Dihydrofolate Reductase (DHFR) 21,22. It is usually accepted that DHFR gene can be amplified in DHFR− CHO cells, which consequently leads to amplification of the co-linked target gene, and finally amplification of recombinant protein 23. In general the degree of gene amplification is proportional to the level of gene expression. Although the chromosomal integration site of a recombinant gene has a major effect on its transcription rate, the phenomenon called the "positional effect". In many cases, transgenes are rapidly inactivated (silenced), probably by the influence of the neighboring heterochromatin that makes them inaccessible to transcriptional machinery 24,25. However, one of the first steps in enhancing gene expression is increasing gene copy number.
One key problem in mAb therapy is the high cost of research, development and production. Scaling-up of capacity, new alternatives (e.g. microbial, eukaryotic or plant-derived hosts) or achieving high producer cell lines may allow much cheaper production of antibodies 26,27. Another alternative is biosimilars, a biological product that is highly similar to a licensed biological product not withstanding minor differences in clinically inactive components, and for which there are no clinically meaningful differences between the biological product and the reference product in terms of the safety, purity, and potency of the product.
The patents on Herceptin are set to expire in Europe in July 2014 and in the US in June 2019. Many pharmaceutical companies such as Biocon-Mylan partnership and Synthon, Amgen and Watson pharmaceuticals in the US and Europe, Samsung and LG Life Sciences in Korea and some pharma companies in India are working on their own biosimilar versions of trastuzumab. So, it looks like there will be no shortage of competition once the patents on Herceptin expire.
Celltrion obtained approval to conduct stage 1 and 2 clinical studies for its biosimilar product of Herceptin on August 31, 2009. According to local news reports, Celltrion is attempting to launch its biosimilar product of Herceptin in 2012. On the other hand, Green Cross obtained approval to conduct the stage 1 clinical study for a bio-better product of Herceptin on 1st October 2010. Green Cross partnered with Maryland, U.S.-based MacroGenics to develop MacroGenics' bio-better product of Herceptin, which is effective to patients who are not responsive to Herceptin, and plans to launch the product in 2016. This work is the first effort for producing a biosimilar version of Herceptin in our country.
Conclusion :
During this study, in total 18 transformants were obtained which had completely integrated the HC and the LC genes into their genomes. Further study, using SDS-PAGE and Western blotting, showed that expression of the HC was much lower than the LC. Moreover, genomic amplification with MTX gave transformants with high level of expression and secretion of the HC and the LC. The real time PCR showed three-fold increase in gene copy number for the HC gene during seven rounds of genomic amplification using MTX. The present study showed that a slight increase in gene copy number (about three times) can increase the level of protein expression up to 1000 times. This may be caused by the neighboring gene effects and the positional effect phenomena 28. The resulting cells could produce mAb with similar biological activity as Herceptin.
Acknowledgement :
We are very grateful to Dr. K. Azadmanesh for Flow cytometry analysis. We are also thankful to Dr. M. Maleki for reviewing the manuscript. This study was supported by a grant from Kawsar Human Genetics Research Center and Kawsar Biotech Co. (Tehran, Iran).
Figure 1. A clonal cell line that was derived from a single cell on semi-solid cloning matrix
|
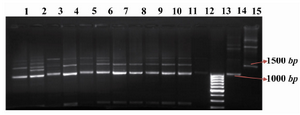
Figure 2. Multiplex PCR. Lanes 1-11: Multiplex PCR products of STD 3-6 and 8-14 genomic DNA. Lane 12: untransfected CHO DG44 cell line as negative control. Lane 13: GeneRuler™ 100bp DNA Ladder (Fermentas). Lane 14: pSLO7 plasmid as positive control for the light chain. Lane 15: pSHC12 plasmid as positive control for the heavy chain
|
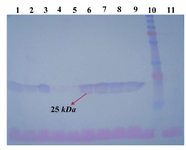
Figure 3. Western blotting using anti-human IgG1 antibody on PVDF membrane. Lanes 1-9: concentrated supernatant of STD 12-18 and 20-21 transformants. Lane 10: PageRuler™ plus pre-stained protein ladder (Fermentas). Lane 11: concentrated supernatant of untransfected CHO DG44 cell line as negative control
|
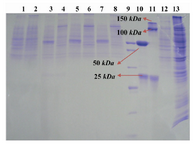
Figure 4. Genomic amplification with methotrexate. Lanes 1 and 2: concentrated supernatant of STD7 transformant in reducing and non-reducing conditions. Lanes 3 and 4: concentrated supernatant of STD72G transformant in reducing and non-reducing condition. Lanes 5 and 6: concentrated supernatant of STD76G transformant in reducing and non-reducing condition. Lanes 7 and 8: concentrated supernatant of STD77G transformant in reducing and non-reducing condition. Lane 9: PageRuler™ unstained broad range protein ladder (Fermentas). Lanes 10 and 11: Herceptin in reducing and non-reducing condition as positive control. Lane 12: culture supernatant from untransfected CHO DG44 cell line as negative control. Lane 13: cell lysate of STD77G construct
|

Figure 5. Flow cytometry. Cell lines staining; A, A') CHO DG44; B, B') MDA-MB-361; C, C') MCF7; D, D') MDA-MB-468; E, E') SK-BR-3; F, F') SK-OV-3; G, G') T-47D and H, H') MDA-MB-453) using the anti-human IgG FITC conjugate antibody. The treatment were performed using produced purified Trastuzumab antibody (A-H figs) as experiment (blue-dash lines), Herceptin antibody (A'-H' figs) as positive control (blue-dash lines) and anti-human IgG1 (A-H and A'-H' figs) as isotype-matched control antibody (black lines)
|
|