HER-3 Knocking Down Induces G2/M Arrest in Gastric Cancer Cells
-
Mokhtari, Ehsan
-
Department of Biology, Faculty of Biological Sciences, Islamic Azad University, East Tehran Branch, Tehran, Iran
-
Mokhtari, Hesamodin
-
International Campus, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
-
 Moslemi, Elham
Department of Biology, Faculty of Biological Sciences, Islamic Azad University, East Tehran Branch, Tehran, Iran , Tel/Fax: +98 9123355872, +98 21 88194061
Moslemi, Elham
Department of Biology, Faculty of Biological Sciences, Islamic Azad University, East Tehran Branch, Tehran, Iran , Tel/Fax: +98 9123355872, +98 21 88194061
-
Department of Biology, Faculty of Biological Sciences, Islamic Azad University, East Tehran Branch, Tehran, Iran
Abstract: Background: The Human Epidermal growth factor Receptor-3 (HER-3) is a member of ErbB receptor family and has deficient kinase activity. HER-3 should heterodimerize with other members of ErbB receptor family, especially with HER-2, to transduce downstream signaling pathways. HER-3 co-expresses with other ErbB receptors in different cancers and overexpresses while the oncogenic signaling pathways such as Jak/Stat, MAPK, and PI3K/Akt are activated and promoted. Here, the expression level of HER-3 was evaluated in Iranian gastric adenocarcinoma's patients and the effects of HER-3 knocking down was investigated on cell cycle and cell viability of human gastric adenocarcinoma cell line of MKN45.
Methods: In this study, 38 paraffin-embedded surgical adenocarcinoma specimens and their marginal non-tumor tissue samples were collected. Total RNAs were extracted and cDNAs were synthesized. Finally, the expression level of HER-3 was evaluated by real time PCR approach. Moreover, the human adenocarcinoma cell line of MKN45 was transfected with siRNA against HER-3 and the effects of its down-regulation were evaluated using MTT assay and cell-cycle analysis.
Results: The data obtained from this study revealed HER-3 is significantly overexpressed in gastric tumors rather than non-tumor marginal tissues. Also, it was found that the expression level of HER-3 is elevated with tumor depth of invasion. Moreover, HER-3 knocking down promotes cell accumulation in G2/M phase of cell cycle and decreases cell viability in MKN45 cells which suggests a potential role for HER-3 in gastric adenocarcinoma tumorigenesis.
Conclusion: Taken together, these results emphasize the importance of HER-3 receptor in diagnosis and prognosis of gastric adenocarcinoma.
Introduction :
The human Gastric Cancer (GC) is the fourth most commonly diagnosed cancer and the second most common cause of cancer related death worldwide 1,2. Gastric carcinogenesis has been known to be a multistep and multifactorial process. Recent advances in molecular medicine have not only shed light on the carcinogenesis of gastric cancer, but also offered novel approaches regarding prevention, diagnosis and therapeutic intervention 3. Recently, HER-3, a member of receptor tyrosine kinases, has been introduced as a new biomarker in diagnosis and prognosis of gastric cancer in human 4.
The human Epidermal Growth Factor Receptor (EGFR) is the most studied family of Receptor Tyrosine Kinases (RTKs) that have important roles in signal transduction and carcinogenesis 5. ErbB receptors are transmembrane receptors consisting of four proteins including HER1/EGFR/ErbB1, HER2/ NEU/ErbB2, HER-3/HER-3 and HER4/ErbB4 6.
Two mechanisms promote receptor dimerization including direct binding of ligands and high receptor density due to overexpression 7. There is a large number of EGFR ligands that drive the receptor homo- or heterodimerization 8. HER-3 lacks intrinsic tyrosine kinase activity and it is unable to trigger downstream signaling pathways on its own 9. Therefore, HER-3 dimerizes with other ErbB receptors, specifically with HER-2 to become activated 10.
Of all the dimers formed by members of the ErbB receptor family, HER-2/HER-3 heterodimers are the most preferable activators of downstream signaling 10,11. The importance of HER-2 receptor in some cancers such as breast and gastric cancers has been verified in a lot of recent studies, whereas there are few reports focused on HER-3 as a significant factor involved in carcinogenesis 12. HER-3 is widely expressed in brain, prostate, kidney, lung, spinal cord and mammary glands and its expression is about 50% higher in cancer cells in comparison with normal cells 13. There are new evidences indicating that HER-3 overexpression is related to tumor size and increased metastasis 14. New researches focused on molecular mechanisms of function of HER-3 in carcinogenesis 13. The elevated expression of HER-3 confers chemo drug resistance in HER-2+ breast cancer cells through activation of PI-3 K/Akt signaling pathway 15,16. All of the mentioned evidences imply the important role of HER-3 receptor in tumor initiation and progression, and also chemo-resistance 14. Some recent studies considered that HER-3 is highly expressed in gastric cancer. It is believed that inhibition of HER-3 signaling with new recombinant drugs may be necessary to overcome chemo-resistance and efficient cancer treatment 17. Therefore, considering the importance of HER-3 in tumorigenesis, an attempt was made to evaluate the expression level of HER-3 gene in Iranian populations suffering from gastric cancer. In addition, HER-3 was knocked down by siRNA in human gastric cancer cell line of MKN45 and its effects on cell cycle and cell viability were evaluated by flow cytometry and MTT assay, respectively.
Materials and Methods :
Clinical samples collection: Human tumor and marginal non-tumor (normal tissues obtained from the margin of same tumors were used as controls) paraffin-embedded surgical specimens from 38 patients with gastric cancer specimens were kindly provided by Masoud Pathobiology Laboratory (Tehran, Iran). Twenty-six samples belonged to males and twelve samples were for females. Patients' ages were in the range between 31 to 73 years old with average age of 54. According to tumor location, 22 samples were non-cardia and 16 samples were cardia tumor and based on grade of differentiation, the low, intermediate and high grades were 11, 13, and 14, respectively (Table 1). The stages and grades were confirmed with histopathological parameters according to WHO criteria.
Cell culture: MKN45, a human gastric adenocarcinoma cell line, was provided by Pasteur Research Institute of Tehran, Iran. Cells were cultured in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal calf serum, 100 U/ml penicillin and 100 U/ml streptomycin (Gibico, CA) and incubated at 37°C with 5% CO2.
RNA extraction and cDNA synthesis: 4 to 6 sections (each section in the size of 10 µm) were prepared for each block of paraffinized specimen by microtome. Xylene and alcohol were used for deparaffinization and tissues were treated with proteinase K (Fermentase, Lithuania). Each pellet was separately treated with RNX plus solution (CinnaGen, Iran) according to the instruction of manufacturer. Quality and quantity of extracted RNAs were evaluated by UV spectrophotometry (260/280 nm ratio). RNAs were treated with DNaseI (Fermentase, Lithuania) to eliminate any unwanted DNA contamination. The first strand of cDNAs was synthesized using the reverse transcription system (TaKaRa, Japan). Non-reverse transcription (No-RT) control samples were acquired simultaneously from DNase treated RNA to find out any potential genomic DNA contamination.
siRNA and cell treatment: HER-3 siRNA duplexes were synthesized by Qiagen (Valencia, CA, USA). The targeted sequences (sense strand) were as follows: HER-3: 5ꞌ-AACCAATACCAGACACTGTAC-3ꞌ. For most experiments, approximately 2×105 cells were plated in six-well plates in medium containing 10% FCS and cultured for 24 hr and then 75% confluence cells were transfected with siRNA using Lipofectamine 2000 (Invitrogen, Paisley, UK) as recommended by manufacturer's instruction. The cells were harvested 0, 24, 48, and 72 hr after transfection by siRNA.
Real-time PCR assay: The expression level of HER-3 gene in tumor and marginal non-tumor samples was investigated by specific HER-3 primers: F; 5ʹ-AGTGAGGCCAAGAC TCCAAT-3ʹ, R; 5ʹ-ACTCCCAAACTGTCACACCA-3ʹ. Also, GAPDH gene was used as an internal control and its primer sequences were as follows: GAPF; 5ʹ- ATGGAGAAGGCTGGGGCT-3ʹand GAP R; 5ʹ-ATCTTGAGGCTGTTGTCATACTTCTC-3ʹ.
Quantitative real time PCR was performed with SYBR green master mix based on manufacturer’s instruction. The PCR program was as follows: initial denaturation at 95°C for 10 min, followed by 40 cycles of denaturation at 95°C for 10 s, and annealing/ extension at 60°C for 30 s. The PCR product size verified by running on agarose gel and the authenticity of amplified fragment were confirmed by direct sequencing of PCR product.
MTT assay: MNK45 cells were seeded at a density of 4×103 cells/well on a 96-well plate. After transfection with siRNA against HER-3 and scrambling, viability of cells was investigated after 48, 72 and 96 hr by MTT assay. MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] was added to each well at a concentration of 500 µg/ml, and plates were incubated for 4 hr at 37°C. Then, supernatants were removed and cells were lysed with 400 µl DMSO. Cells were incubated further for 10 min at 37°C with gentle shaking. Absorbance was measured at 570 nM using a computer-controlled microplate analyzer.
Cell cycle analysis: Cells were seeded in 6 well plates with density of 2×105 cells/well and incubated at 37ºC overnight. Then, cells were transfected with HER-3 siRNA, scrambled and incubated for 48 hr. Transfected cells were harvested and fixed by 70% cold ethanol at 4°C overnight and further analyzed by flow cytometry using PI/RNase staining buffer for cell cycle analysis. Distribution of cells in various cell phases was analyzed by Flowing Software 2.5.1.
Western blotting analysis: After siRNA treatment, MNK45 cells were lysed in 1 ml lysis buffer composed of 50 mM Tris-HCL, pH=7.4, 5 mM EDTA, 1% Triton X-100, 150 mM NaCl and 1% protease inhibitor cocktail for 1 hr on ice with 15 min interval of vortexing for 30 s. Using protein assay kit (Bio-Rad Laboratories, CA), the protein concentrations were measured. 30 µg of cell lysates were used for SDS-PAGE. Then, resolved proteins were transferred onto PVDF membrane. Blocking was performed for overnight at 4oC with 5% non-fat milk in PBS plus 0.05% Tween 20. After three times of washing with PBS-Tween with 3% non-fat milk, PVDF membrane was incubated with 5 µg of anti-HER-3 monoclonal antibody (Abcam, Cambridge, UK) for 1 hr at room temperature. After three times of washing with PBS-Tween, membrane was incubated with Horseradish Peroxidase (HRP)- conjugated beta-actin antibody (Abcam, Cambridge, UK) for 1 hr at room temperature followed by washing and developing with ECL chemiluminescence detection system (Pierce ECL Substrate Western blot detection system, Thermo Scientific, IL) and was exposed to autoradiography film (Kodak XAR film).
Statistical analysis: To calculate relative expression of HER-3 in patients, 2-∆∆Ct formula was used. Differences in gene expression between two groups in each analysis were evaluated using one-sample t-test which was performed by SPSS ver. 20 and histograms drawn using Graph Pad Prism 6 Demo software package. The results are expressed as mean±SE of at least triplicate independent experiments. Comparisons between two groups were performed by Mann-Whitney U test. The comparison of HER-3 expression between various clinical characteristics was analyzed by the chi-square test. A p-value less than 0.05 was considered statistically significant.
Results :
HER-3 is overexpressed in human gastric adenocarcinoma: Using specific primers, the expression level of HER-3 was evaluated in tumor and non-tumor marginal tissues and between different grades of gastric adenocarcinoma. The results of this study manifested that HER-3 is expressed in both tumor and non-tumor marginal samples of stomach. However, the level of expression is significantly higher in tumor samples compared to non-tumor marginal samples (p=0.01). Moreover, in the tumor group, HER-3 expression was significantly associated with depth of invasion (p=0.023) (Figure 1).
HER-3 RNA and protein levels reduced after transfection of siRNA: As described in materials and methods, MKN45 cells were transfected with siRNA against HER-3 and scrambled control. After 72 hr, HER-3 mRNA level was decreased about 80% compared to the scrambled control. Also, it was found that HER-3 protein level was sharply reduced in siRNA transfected cells.
Suppressing HER-3 by siRNA arrested MKN45 cells in G2/M phase of cell cycle: Gastric adenocarcinoma cell line of MKN45 was transfected with siRNA against HER-3 and scrambled control. After staining cells with PI, distribution of cells in various phases of cell cycle was analyzed by flow cytometry. Our data revealed that cells treated with siRNA were accumulated in G2/M phase of cell cycle in comparison with the scrambled control cells. Also, the number of cells in G1 and S phases was decreased in siRNA treated cells (Figure 2).
HER-3 down-regulation by siRNA decreased viability of MKN45 cells: MKN45 cells were transfected with HER-3 siRNA and prepared for MTT assay after 24, 48 and 72 hr. Our data showed that HER-3 siRNA significantly decreased MKN45 cell proliferation by 50% after 48 hours (Figures 3 and 4).
Discussion :
Gastric adenocarcinoma is an aggressive cancer and remains the fourth most common type of cancer and is the second leading cause of cancer-related death worldwide. Gastric cancer is often diagnosed at an advanced stage. The most common therapy is surgical resection in company with chemotherapy or chemo-radiation in appropriate cases 18. Unfortunately, treatment of advanced or metastatic gastric cancer has seen little progress and median Overall Survival (OS) in this group remains <1 year 19. Gastric cancer is a heterogeneous disease that demands continued attention and research with regard to prevention, early detection and novel therapeutic options. Therefore, finding specific markers for early diagnosis of this cancer is very important in early treatment and preventing more cancer progress.
The human HER-3 is detected in tissues such as brain, prostate, mammary gland, lung, spinal cord, kidney, and liver 13. There are increasing reports for HER-3 role in onset and development of cancer. HER-3 can become activated with direct binding of ligands such as Neuregukin-1 (NRG-1) and Neuregulin-2 (NRG-2), but its intrinsic kinase domain is deficient and it is therefore unable to trigger downstream signaling pathways on its own 20-22. Thus, HER-3 receptor should dimerize with other ErbB receptors, especially with HER-2, to drive downstream signaling pathways. One of the requisites for tyrosine kinase activity of HER-2 is the contribution of HER-3 receptor 14,22. Several signaling pathways can be activated by hetero-dimerization of HER-3 with other members of ErbB family including PI3K/Akt, PLCγ/PKC, Jac/Stat and MEK/MAPK. Of all dimers formed by the members of ErbB receptor family, HER-2/HER-3 heterodimers are the most potent activator of downstream signaling 14,22. Interestingly, the cells that lack HER-3 receptors or only a few of them, cannot proliferate and these anti-proliferative properties resulted from loss of HER-3 could not be compensated by HER-1 or HER-4 partners, which suggests the great importance of HER-3 in carcinogenesis 10. HER-2/HER-3 heterodimers induce cell proliferation and inhibit apoptosis mainly by enhancing PI3K/Akt signaling pathway. The PI3K/Akt pathway is involved in survival and activated in many cancers. In fact, direct binding of HER-3 to PI3K enhances PI3K/Akt pathway induced by HER-2/HER-3 heterodimers 10,20,23. Recently, the role of HER-3 in primary tumors and acquired resistance of HER-2+ breast cancer cells to chemo drug therapy has attracted serious attentions 24. HER-3, as a major cause of treatment failure in chemotherapy, mainly confers its influences through activation of PI3K/Akt signaling pathway 25.
Several oncogenic mutations in HER-3 gene have been detected in gastric and colon cancers that elucidate the role of HER-3 in development of cancer 26. Jeong et al detected protein- altering mutations in HER-3 in 1% of patients with colon cancer 27. Jaiswal et al identified HER-3 somatic mutations in 11% of patients with colon cancer and 12% patients with gastric cancer 12. Wang et al reported frequent mutations in the HER-3 gene in 10% of patients with gastric cancer 28. Based on a meta-analysis by Chen et al, HER-3 plays an essential role in the clinicopathology and prognosis of gastric cancer 29.
All the above mentioned observations suggest that HER-3 is the most important partner of HER-2 in HER-2 positive breast cancer and greater attentions must be paid on its molecular functions in chemo-resistance and carcinogenesis. Since, the overexpression of HER-3 has been observed in some cancers such as breast and gastric adenocarcinoma, in this study, the expression level of HER-3 was evaluated in Iranian patients with gastric adenocarcinoma. Obtained data in this study demonstrated that the expression of HER-3 gene is elevated in gastric tumors compared to non-tumor tissues. Notably, the average expression level of HER-3 gene was higher in T3-T4 classified tumors. Also, HER-3 knocking down by siRNA reduced cell viability and accumulated cells in G2/M phase of cell cycle.
Osaki et al and Zhang et al showed that HER-3 causes uncontrolled cell cycle by PI3K/Akt signaling pathway 30,31. In our results, G2/M arrest occurred in MKN45 cells by silencing of HER-3. Accumulation of cells in G2/M phase by HER-3 siRNA is probably related to the Akt inactivation by decreasing cyclin B1 that is resulted in cell proliferation inhibition.
Our results confirmed Wu et al’s results that showed HER-3 is involved in cell proliferation and viability in gastric cancer cell lines 32. Taken together, these findings suggest a potential correlation between HER-3 expression level and gastric tumor progression and malignancy.
Conclusion :
Our study demonstrates the importance of expression level of HER-3 member for gastric cancer diagnosis and prognosis. This study might provide further insight into the prevention and detection of gastric cancer in early stages in near future.
Acknowledgement :
This work was financially supported by Islamic Azad University, East Tehran Branch. The authors would like to thank Masoud Pathobiology Laboratory for cooperation in gastric tumor sample obtaining. There is no conflict of interest in this study.
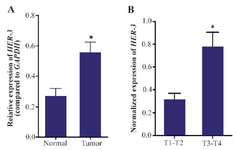
Figure 1. Real time PCR analysis of HER-3. The expression of HER-3 is up-regulated in gastric tumors compared to the non-tumor marginal samples (p-value=0.01). Moreover, the expression level of HER-3 gene is elevated with tumor depth in gastric adenocarcinoma (p=0.023). Real-time PCR was performed as duplicates for three times for each sample. The expression values were normalized relative to the expression level of GAPDH as a housekeeping gene.
|
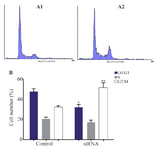
Figure 2. Cell cycle analysis of MKN45 cell lines using flow cytomtery. MNK45 cells were treated with HER-3 siRNA (A1), scrambled (A2) and analyzed by flow cytometry after 48 hr. B) number of cells distributed in different cell cycle phases in cells transfected with siRNA and scrambled. HER-3 silencing increased cell accumulation in G2/M phase of cell cycle up to 20% compared to the scrambled-treated control cells (p= 0.029).
|
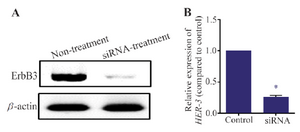
Figure 3. Western blotting assay for MKN45 cells. MKN45 cells were transfected with HER-3 siRNAs and harvested 72 hr after transfection. The cells were lysed and 50 mg of total protein was used for detection of ErbB3 by western blotting. HER-3 protein level was decreased after treatment with siRNA. Beta-actin was used as internal control of western blot.
|
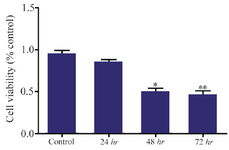
Figure 4. Cell viability analysis of siRNA treated MKN45 cells by MTT assay. MKN45 cells were harvested 24, 48 and 72 hr after ErbB3 siRNA transfection and analyzed by MTT assay. Our results showed that ErbB3 downregulation reduced cell viability by 50%, 48 hr after treatment with siRNA compared to scrambled control (p-value=0.013).
|
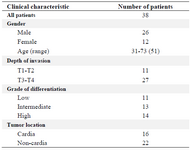
Table 1. Pathological characteristics of patient’s sample
|
|