Green Tea Extract Reduced Lipopolysaccharide-Induced Inflammation in L2 Cells as Acute Respiratory Distress Syndrome Model Through Genes and Cytokine Pro-Inflammatory
-
Priyandoko , Didik
-
Biology Study Program, Faculty of Mathematics and Natural Sciences, Indonesia University of Education, Bandung 40154, Indonesia
-
 Widowati, Wahyu
Faculty of Medicine, Maranatha Christian University, Bandung 40164, Indonesia, Tel: +62 819 10040010; E-mail: wahyu_w60@yahoo.com
Widowati, Wahyu
Faculty of Medicine, Maranatha Christian University, Bandung 40164, Indonesia, Tel: +62 819 10040010; E-mail: wahyu_w60@yahoo.com
-
Lenny , Lenny
-
Faculty of Biotechnology, Atma Jaya Catholic University of Indonesia, BSD Campus, Tangerang 15345, Indonesia
-
Novianti, Sintya
-
Faculty of Biotechnology, Atma Jaya Catholic University of Indonesia, BSD Campus, Tangerang 15345, Indonesia
-
Revika, Revika
-
Faculty of Biotechnology, Atma Jaya Catholic University of Indonesia, BSD Campus, Tangerang 15345, Indonesia
-
Adhani Sholihah, Ika
-
Biomolecular and Biomedical Research Center, Aretha Medika Utama, Bandung 40163, Indonesia
-
School of Life Sciences and Technology, Bandung Institute of Technology, Bandung 40163, Indonesia
Abstract: Background: Acute Respiratory Distress Syndrome (ARDS) is a severe lung inflammatory condition that has the capacity to impair gas exchange and lead to hypoxemia. This condition is found to have been one of the most prevalent in patients of COVID-19 with a more serious condition. Green tea (Camellia sinensis L.) contains polyphenols that possess many health benefits. The purpose of this study was to assess the anti-inflammatory activities of green tea extract in Lipopolysaccharide (LPS)-induced lung cells as ARDS cells model.
Methods: In this study, rat lung cells (L2) were induced by LPS to mimic the inflammation observed in ARDS and later treated with green tea extract. Pro-inflammatory cytokines such as Interleukin (IL)-12, C-Reactive Protein (CRP) as well as Tumor Necrosis Factor-α (TNF-α) were investigated using the ELISA method. Gene expression of NOD-Like Receptor Protein 3 (NLRP-3), Receptor for Advanced Glycation End-product (RAGE), Toll-like Receptor-4 (TLR-4), and Nuclear Factor-kappa B (NF-κB) were evaluated by qRTPCR. Apoptotic cells were measured using flow cytometry.
Results: The results showed that green tea extract treatment can reduce inflammation by suppressing gene expressions of NF-κB, NLRP-3, TLR-4, and RAGE, as well as pro-inflammatory cytokines such as IL-12, TNF-α, and CRP, an acute phase protein. Apoptosis levels of inflamed cells also found to be lowered when green tea extract was administered; thus, also increasing live cells compared to non-treated cells.
Conclusion: These findings could lead to the future development of supplements from green tea to help alleviate ARDS symptoms, especially during critical moments such as the current pandemic.
Introduction :
Systemic inflammation of the lungs of the body causes Acute Respiratory Distress Syndrome (ARDS). ARDS is often associated with diseases such as traumatic brain injury, burn injury, immune system systemic suppression, and bacterial or viral infections, as recently found out in the case of SARS-CoV-2 1-3. ARDS is characterized by very poor oxygenation, reduced pulmonary infiltrates, and bilateral radiographic infiltrates 4. Various studies have shown that the death of patients with ARDS is common 5. Previous studies have shown that there are similarities between the pathological anatomy of patients who died from COVID-19 and ARDS. The pathological anatomy of ARDS can aid in the understanding and treatment of COVID-19 patients 6.
ARDS is usually caused and worsened by a phenomenon known as the Cytokine Storm Syndrome (CS). CS is a phenomenon in which the immune cells, as a direct response to injury or pathogens, release a huge amount of pro-inflammatory cytokine and chemokine like Interleukin (IL)-6, IL-12, IL-1β, and Tumor Necrosis Factor-α (TNF-α) in an attempt to attract other immune cells to migrate to the site of the injury 5,7,8. Moreover, C-Reactive Protein (CRP), which is also released in reaction to inflammation and primarily functions to aid in opsonization as well as the release of other pro-inflammatory cytokines, is also produced 9. The genes that are responsible for producing and playing a role in its cascade in the inflammatory pathways are also diverse. These inflammatory cytokines would then be recognized by receptors which are regulated by genes such as Toll-Like Receptor-4 (TLR-4) and Receptor for Advanced Glycation End products (RAGE) 10. The gene that is also responsible for the production of cytokines would include Nod-Like Receptors-3 (NLRP-3) which also plays a role in the pathway of "Nuclear Factor-kappa B (NF-kB) 11,12. Accordingly immune cells, whether innate or adaptive, will migrate and destroy the inflammation trigger and protect infected or injured cells 13,14. At an appropriate amount, these immune cells will help to cure, but when there is too much destruction our body will not be able to handle it. Especially in the case of ARDS where the destruction happens in one of the most critical tissues in the body, the alveoli which help with gas exchange. Once the epithelial cells are destroyed, leakage of high-protein fluids will lead to edema. Over time, this edema can cause hypoxia due to its impact on gas exchange 1,3.
Treatment for patients usually include the usage of low tidal mechanical ventilators in hospitals. However, other than synthetic drugs such as simvastatin, and in the case of COVID-19, methylprednisolone and dexamethasone, an alternative cure has yet to be found 15,16. The problem with synthetic drugs is that they often cause negative side consequences. Non-Steroidal. Anti-Inflammatory Drugs (NSAIDs), generally also used to cure inflammations, are known to increase the risk of gastrointestinal illnesses like dyspepsia and esophagitis 17,18. Various plants and herbs have been investigated as the source of anti-inflammatory agents. Tea (Camellia sinensis L.) is a renowned plant that contains large amounts of polyphenols and can act as an anti-inflammatory agent. Tea contains several types of compound groups such as phenolics, alkaloids, polysaccharides, and saponins 19. The main polyphenol compound in green tea that is thought to help with its anti-inflammation activities are flavonoids such as quercetin and myricetin. A previous study has also detected 8-prenylnaringenin (8-PN) and 6-prenylnaringenin (6-PN) in the Green Tea Extract (GTE) used 17,20.
The aim of this study was to evaluate the effect of GTE on Lipopolysaccharide (LPS)-induced lung cells as ARDS cells model by measuring gene expression of NF-κB, NLRP-3, RAGE, and TLR-4 by RT-PCR and examination of proinflammatory cytokine such as cytokines TNF-α, CRP, and IL-12 using sandwich Enzyme-Linked Immunosorbent Assay (ELISA). Flow cytometry was utilized to examine the apoptotic activity of the cells to determine their condition.
Materials and Methods :
Green tea extract preparation: The GTE was processed by PT. Fathonah Amanah Shidiq Tabligh (Depok, Indonesia) (Batch No. 00107201057) and the process based on Good Manufacturing Practice (GMP) standard by The Indonesian Food and Drug Authority (BPOM). The extraction process of GTE used 70% ethanol as its solvent. Before GTE was stored at 25±2 °C, lactose was added to GTE first for getting the powder form.
L2 cells-LPS induced as ARDS cells model: Rat lung (L2) cells (ATCC® CCL-149TM) was obtained from the Biomolecular and Biomedical Research Center, Aretha Medika Utama which is located in Bandung, West Java, Indonesia. T75 flask with a complete medium was used for cell growth until confluent. The complete medium composition was Dulbecco's Modified Eagle’s Medium (DMEM) High-glucose (Biowest, L1003-500), 10% Fetal Bovine Serum (FBS) (Biowest, S1810-5000), 1% Antibiotic-antimycotic (ABAM) 100× (Biowest, L0009), and 0.1% Gentamicin (Gibco, 15750078). Confluent cells were induced with 4 µg/ml LPS Escherichia coli O55:B5 (Sigma-Aldrich L2880) to produce an inflammation model and incubated for 18 hr at 37°C in a 5% CO2 incubator. Various concentrations of GTE (1.56, 3.13, and 6.25 µg/ml) were applied to the cells and incubated for 24 hr in a 5% CO2 incubator (37°C). The levels of IL-6, CRP and TNF-α in the harvested supernatant cells were measured using the ELISA method, while the cell pellets were collected for RNA isolation to be used for testing TLR-4, RAGE, NF-κB, and NLRP-3 gene expression using qRT-PCR 8.
Quantification of TNF-α, CRP, and IL-12 levels in the L2 cells using ELISA: The supernatant cells were used to determine the levels of pro-inflammatory cytokine expression in the L2 cells, such as TNF-α, CRP, and IL-12. The TNF-α, CRP, and IL-12 levels were determined by the ELISA kit (Elabscience E-EL-R0019, E-EL-R0506, and E-EL-R0064, respectively). The process was carried out according to the manufacturer's guidelines. The sample absorbances were tested at a wavelength of 450 nm 8.
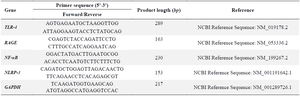
Quantification of NF-κB, NLRP-3, RAGE, and TLR-4 gene expression in the L2 cells using qRTPCR: The levels of NF-κB, NLRP-3, RAGE, and TLR-4 gene expression were determined from pellet cells. Direct-zol RNA Miniprep Plus Kit (Zymo, R2073) was applied to isolate total RNA, the procedure was according to the protocol of manufacture. Then, the SensiFAST cDNA synthesis kit was used to synthesize complementary DNA and the procedure was according to the protocol of manufacture. AriaMx 3000 Real-Time PCR System (Agilent) was used to measure quantitative gene expression, primer sequence (Table 1), and Evagreen master mix (Bio-Rad, 1725200) for the reaction mixture. The procedure was undertaken based on the manufacturer's protocol 21. Real-time PCR was run for 40 cycles using GAPDH as reference gene, and the annealing temperatures was 54°C, 56°C, and 57°C for TLR-4, RAGE, and NF-κB, NLRP-3, respectively.
Apoptosis assay using flow cytometry analysis: Annexin V-FITC/PI Apoptosis Detection Kit (Elabsci, E-CK-A211) was utilized to assess the cells apoptosis in line with the manufacturer's instructions, with slight modification. Cells incubated for 2 hr on a 6-well plate (n=500,000). After 4 days, the growth medium was removed, harvested and centrifuged at 1,600 rpm for 5 min, and then 500 μl FACS buffer added on pellet and centrifuged. Additionally, annexin and Propidium Iodide (PI) staining was performed on the pellets after the addition of 100 μl FACS buffer. MACSQuant Analyzer. 10 Flow Cytometer (Miltenyi, Bergisch Gladbach, Germany) was used to evaluate the stained cells that have been incubated for 1 hr 22.
Statistical analysis: The data analysis was conducted using SPSS 26.0 software, and experimental results are shown as mean±SD. The comparison between groups was assessed using a one-way ANOVA and the post hoc test was Dunnett's test and Tukey HSD test with p<0.05. Analysis using Kruskal-Wallis and Mann-Whitney. post hoc with p<0.05 was used for non-parametric.
Results :
The influence of GTE on TNF-α, CRP, and IL-12 levels: Pro-inflammatory cytokine was increased by LPS induction. Figure 1 shows the effect of GTE toward IL-12, TNF-α, and CRP levels. Compared to the negative control, the level of IL-12, TNF-α, and CRP increased significantly in positive control (p<0.05). Treatment with GTE on L2 cells reduced pro-inflammatory cytokine levels compared to positive control. The results showed that GTE reduced CRP and TNF-α levels significantly in the rat lung cell at concentration of 1.56 µg/ml (p<0.05). Meanwhile, the IL-12 level did not show any significant reduction in all concentration of GTE.
Effect of GTE toward TLR-4, RAGE, NF-κB, and NLRP-3 genes expression: LPS-induced L2 cells significantly increased TLR-4, RAGE, NF-κB, and NLRP-3 genes expression (p<0.05). As shown in figure 2, GTE treatments downregulated the expression of TLR-4, RAGE, NF-κB, and NLRP-3 genes expression ((p<0.05). The results showed that TLR-4, RAGE, and NLRP-3 genes expression were significantly decreased with GTE treatment at the concentration of 1.56 µg/ml (p<0.05). In contrast, the 6.25 µg/ml GTE showed the most effective concentration in suppressing NF-κB gene expression but did not show any significant difference with the positive control.
Effect of GTE toward cells apoptosis: The effect of GTE was analyzed by flow cytometry in triplicate using concentrations of 6.25, 3.13, and 1.56 µg/ml respectively. Figure 3 shows that LPS induction increased the number of cells in necrosis, early, and late apoptosis, and also reduces the number of live cells quantity compared to the negative control. The dot blot of the effect of GTE on cell apoptosis can be seen in figure 4. The number of necrosis, early and late apoptosis were decreased following GTE treatment, while the number of viable cells increased.
According to figure 4, the GTE treatment increased the percentage of live cells compared to the positive control. Moreover, depending on the concentration, treatments with GTE decreased the percentage of necrosis cells with concentration dependent manner, and GTE treatment reduced the percentage of apoptotic cells. The highest GTE concentration (6.25 µg/ml) had the smallest apoptosis percentage compared to other GTE treatments with the apoptosis percentage of 10.34%.
Discussion :
One of the life-threatening illnesses observed in severely ill COVID-19 patients is ARDS. ARDS, just like any other inflammation can be induced by different agents such as protein in viruses or LPS of bacteria. LPS administration in both in vivo and in vitro leads to inflammation and causes the release of inflammatory cytokines 23,24. LPS induction led to an increase in CRP levels as well as pro-inflammatory cytokines as TNF-α and IL-12, as shown in figure 1. This is also proven by a previous study, who also found that LPS induction releases uncontrolled cytokines that could lead to lung injury 25.
In order to compare the cytokine or expression levels of inflamed cells with those of healthy cells, negative controls were required for all assays in which LPS or the extract was not added to the cells. Additionally, both positive and DMSO controls were established. To confirm that the solvent for the GTE had no significant effect on the inflamed cells, DMSO was used as a control. This would allow us to conclude that the difference in results between treated and untreated cells was due to the GTE treatment and not the solvent used.
In the absence of a stimulus, healthy cells should have lower levels of pro-inflammatory cytokines and expression of genes related to inflammation than inflamed cells 26. When pro-inflammatory triggers such as LPS are identified, pro-inflammatory cytokines begin to be produced. In both animal models and patient studies, the TLR-4 has been shown to be a receptor for the response to LPS 27. RAGE receptors, receptors highly expressed in skin and lung tissue, are also involved in the LPS response. RAGE expression can be increased in the presence of inflammatory conditions, aging, and at the time of injury. RAGE and TLRs are crucial components of the natural immune system; as these compounds can interact with various microbial products and molecules (one of them is LPS) in inflammation and tissue injury 28. The NF-κB signaling pathway can be activated by either TLR-4 or RAGE receptors. Activated NF-B controls the synthesis of proinflammatory cytokines and chemokines during inflammation 29. Previous research has shown that NF-κB signaling is involved in activating NLRP-3 30. An immunological complex called the NLRP-3 inflammasome is made up of NLRP-3, an apoptosis-associated protein with a pro-caspase-1 and a C-terminal caspase recruitment domain (ASC) 31. NLRP-3 also recognizes Reactive Oxygen Species (ROS) and Damage-Associated Molecular Patterns (DAMPs) in inflammation cells (LPS-induced cells) 32.
Figures 1-4 show that DMSO controls had insignificant levels of pro-inflammatory cytokines compared to positive controls. Based on the results of DMSO control for the gene expression, levels of TLR4, RAGE, NF-κB, and NLRP3 were comparable with figure 3; where the DMSO control had insignificant results with positive control. Thus, it could be assumed that all the results from treatments are purely based on the treatment of GTE.
Green tea contains many beneficial polyphenols such as catechins, quercetin, myricetin, and kaempferol 33. These polyphenols have made green tea a well-known beverage with many therapeutic benefits and are often used in many traditional therapies. Additionally, green tea has been reported to have antioxidant and anti-inflammatory properties that can help decrease inflammation by lowering inflammatory markers. Its antioxidant activity helps to scavenge ROS which can lead to the suppression of NF-κB activity 34,35. Figure 2 shows that there was an increase in the expression of TLR-4, RAGE, NF-κB, and NLRP-3 genes. LPS induction on human dental pulp stem cells upregulated TLR-4 gene expression 36. The findings of this study demonstrate that TLR-4 is a particular receptor for inducing LPS in cells 37. In addition, in this study, the increase of TLR-4 gene expression was in line with NF-κB gene expression in response to LPS. These results are consistent with previous studies which LPS induction increased NF-κB gene expression 37. Moreover, LPS induction also increases the NLRP-3 expression on RAW 264.7 cells and is in line with the NF-κB expression38. The results of this research indicate that GTE downregulated the expression of TLR-4, RAGE, NF-κB, and NLRP-3 mRNA levels in LPS-induced L2 cells as ARDS cells model.
The findings in figure 2 concur with those of the previous investigations. From figure 2, it can be inferred that GTE has the ability to suppress the mRNA expression of TLR-4, RAGE, NF-κB, and NLRP-3 in the LPS-induced L2 cells as ARDS cells model. The expression of proinflammatory cytokines including TNF-α and IL-12 was also decreased by reducing NF-κB and other gene expressions (Figure 2).
Besides NF-κB, green tea catechin also inhibits the pro-inflammatory mediators' secretion through NLRP-3 inflammasome, a multiprotein complex that activates inflammatory responses 39. In prior research, treatment with quercetin, one of the components in green tea, was shown to be able to decrease both the mRNA level of NLRP-3 and the amount of the pro-inflammatory cytokine 40. Epigallocatechin-3-gallates (EGCG), in particular, was discovered in a different study to have the ability to suppress and lower the expression of TLR-4 and RAGE following a pre-treatment of EGCG in rat LPS-induced inflammation 41-43. Inhibiting those gene expressions also resulted in a reduction of pro-inflammatory cytokines TNF-α and IL-12 43,44.
Precisely in this study, GTE treatment at all three concentrations significantly reduced TNF-α levels as compared to positive controls. These matched previous findings of GTE and their ability to lower inflammation by modulating pro-inflammatory cytokines like TNF-α, is especially due to the presence of flavonoids such as myricetin and quercetin 45. Treatment with 1.56 μg/ml GTE showed the most active to lower TNF-α level compared to other GTE treatments and significantly decreased TNF-α level compared to positive control. Moreover, GTE 1.56 µg/ml also decreased IL-12 level significantly compared to positive control.
The CRP levels were also found to be highly associated to immunological alterations as a marked rise in pro-inflammatory biomarkers 46. Although CRP is mostly produced in the liver, its mRNA has been found in respiratory tract epithelial cells and T-lymphocytes 47. The cytokine storm in ARDS induces macrophage activation syndrome and results in a pro-inflammatory hypercytokinemia profile, which increases CRP generation by hepatocytes 48,49.
Based on the results shown in figure 2C, administration of GTE showed a significantly reduced level of CRP. The decreased level of CRP is in correlation with IL-6 and TNF-α levels. CRP, an acute phase protein, was induced by both IL-6 and TNF-α in inflammation. It was shown that by decreasing these inflammatory cytokines, the level of CRP was reduced 9. As discussed previously, GTE reduced the level of inflammatory mediators in an inflammation cells model. The most effective result was seen in 1.56 μg/ml GTE for all parameters, except for the expression of NF-κB in which 6.25 μg/ml GTE was the most active to decrease NF-kB gene expression. Therefore, further research is required to establish the excellent concentration of GTE for its effectiveness in treating ARDS.
One other marker of inflammation is apoptosis or programmed cell death 50. Apoptosis is very crucial when it comes to the host defense system as it would allow the damaged or infected cells to die and end further damage inside the host 50. There are several ways apoptosis can be activated and in inflammation, apoptosis could happen due to both internal aspects such as mitochondrial dysfunction and stress, along with external factors including induction by LPS 50,51, both of which activate caspase to start the apoptosis 52. This is why in most cases of inflammation the apoptosis level of cells is usually increased 51.
In case of ARDS, the death of a large number of cells could also lead to the destruction of vital tissues, causing what would ideally be a preventative process of a deadly damage rapidly 50,51,53. Apoptosis suppression has played a crucial role in alleviating inflammation symptoms. The result of the apoptosis assay by flow cytometry indicated that all three-concentrations managed to decreases apoptosis levels of inflamed cells as could be seen in the significant difference of live cells compared to the positive controls. The results of the study were consistent with previous study54 which found that quercetin, one of the constituents of green tea can suppress apoptosis in LPS-induced inflammation. Moreover, all GTE concentration treatments showed significantly reduced cell death, early and late apoptosis levels.
Conclusion :
In this study, it is proven that GTE can help reduce the inflammation by modulation of TNF-α, CRP and IL-12 pro-inflammatory marker as well as suppressing inflammation-related genes expression including TLR-4, RAGE, NF-κB, and NLRP-3. Furthermore, GTE was able to reduce the apoptosis in inflamed cells.
Acknowledgement :
Financial assistance was provided by the Minister of Education, Culture, Research, and Technology of the Republic of Indonesia (Penelitian Dasar Unggulan Perguruan Tinggi 2021) under grant number 163/E4.1/ AK.04.PT/2021. The Biomolecular and Biomedical Research Center, Aretha Medika Utama, Bandung, West Java, Indonesia, also provided the laboratory resources and research methodology for this study.
Conflict of Interest :
The authors declare that they have no competing interest.
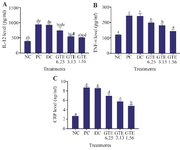
Figure 1. Effect of GTE toward IL-12, TNF-α, CRP on LPS-induced L2 cells. A) IL-12 level (pg/ml) on LPS-induced L2 cells, B) TNF-α level (pg/ml) on LPS-induced L2 cells, C) CRP level (ng/ml) on LPS-induced L2 cells.
* The data is presented as means value ± standard deviation. NC: untreated cell, PC: LPS-induced cell, DC: PC+DMSO1%, GTE 6.25: PC + GTE 6.25 µg/ml, GTE 3.13: PC + GTE 3.13 µg/ml, GTE 1.65: PC + GTE 1.65 µg/ml. A different mark (alphabetical) indicates a significant difference among treatments toward CRP level based on Kruskal-Wallis and Mann-Whitney post hoc test (p<0.05). A different letter (alphabetical) indicates a significant difference among treatments based on Anova and Tukey HSD post hoc test for TNF-α level and Dunnett T3 post hoc for IL-12 level at Anova and Tukey HSD post hoc test p<0.05.
|
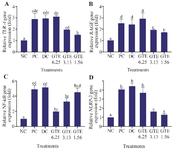
Figure 2. Effect of GTE toward TLR-4, RAGE, NF-κB, and NLRP-3 gene expression on LPS-induced L2 cells. A) TLR-4 gene expression on LPS-induced L2 cells, B) RAGE gene expression on LPS-induced L2 cells, C) NF-κB gene expression on LPS-induced L2 cells, D) NLRP-3 gene expression on LPS-induced L2-cells.
* The data is presented as means value ± standard deviation. NC: untreated cell, PC: LPS-induced cell, DC: PC+DMSO 1%, GTE 6.25: PC + GTE 6.25 µg/ml, GTE 3.13: PC + GTE 3.13 µg/ml, GTE 1.65: PC + GTE 1.65 µg/ml. A different mark (alphabetical) indicates a significant difference among treatments toward RAGE gene expression, based on Kruskal-Wallis and Mann-Whitney post hoc test (p<0.05). A different letter (alphabetical) indicates a significant difference among treatments based Anova and Tukey HSD post hoc test for NLRP-3 gene expression and based Anova and Dunnett T3 post hoc test for TLR-4 and NF-κB gene expression (p<0.05).
|
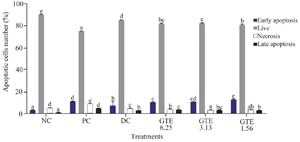
Figure 3. Effect of GTE toward early apoptosis, live cells, necrosis cells, late apoptosis on LPS-induced L2 cells.
* The data is presented as means value ± standard deviation. NC: untreated cell, PC: LPS-induced cell, DC: PC+DMSO 1%, GTE 6.25: PC + GTE 6.25 µg/ml, GTE 3.13: PC + GTE 3.13 µg/ml, GTE 1.65: PC + GTE 1.65 µg/ml. The number of early apoptosis, live, necrosis, and late apoptosis cells was measured in triplicate for each sample. A) Early apoptosis on LPS-induced L2 cells, B) Live cells on LPS-induced L2 cells, C) Necrosis cells on LPS-induced L2 cells, D) Late apoptosis on LPS-induced L2 cells. The data is presented as means value ± standard deviation. NC: untreated cell, PC: LPS-induced cell, DC: PC+DMSO 1%, GTE 6.25: PC + GTE 6.25 µg/ml, GTE 3.13: PC + GTE 3.13 µg/ml, GTE 1.65: PC + GTE 1.65 µg/ml. A different mark (alphabetical) indicates a significant difference among treatments toward early apoptosis, live cells, necrosis cells, late apoptosis based on Anova and Tukey HSD post hoc test (p<0.05).
|
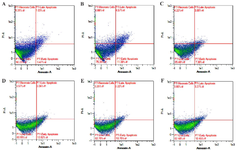
Figure 4. The representative dot blots of different GTE concentrations on LPS-induced L2 cells toward percentage of apoptosis by flow cytometry.
* A) Negative control (untreated cell): live cells 90,23%, early apoptosis 3.47%, late apoptosis 1.03%, necrosis cells 5.28%. B) Positive control (LPS-treated cells): live cells 75.39%, early apoptosis 11.38%, late apoptosis 4.75%, necrosis cells 8.66%. C) DMSO control (untreated cells+DMSO): live cells 85.48%, early apoptosis 7.30%, late apoptosis 3.00%, necrosis 4.22%. D) GTE 1.56 µg/ml: live cells 80.55%, early apoptosis 12.82%, late apoptosis 3.06%, necrosis cells 3.57%. E) GTE 3.13 µg/ml: live cells 82.78%, early apoptosis 10.75%, late apoptosis 3.22%, necrosis cells 3.25%. F) GTE 6.25 µg/ml: live cells 82.48%, early apoptosis 10.45%, late apoptosis 3.27%, necrosis cells 3.08%.
|
|
|