Anti-Quorum Sensing and Anti-Biofilm Activity of Ginger (Zingiber officinale) Rhizomes against Multidrug-Resistant Clinical Isolates of Pseudomonas aeruginosa
-
Kumar Sagar , Pankaj
-
Department of Microbiology, Bundelkhand University, Jhansi-284128, Uttar Pradesh, India
-
Sharma , Poonam
-
Department of Zoology, Indira Gandhi National Tribal University (A Central University), Amarkantak-484886 , Madhya Pradesh, India
-
 Singh, Rambir
Department of Horticulture, Aromatic and Medicinal Plants, Mizoram University (A Central University), Aizawl-796004, Mizoram, India, Tel: +91 9473583251; E-mail: sehrawat_r@yahoo.com
Singh, Rambir
Department of Horticulture, Aromatic and Medicinal Plants, Mizoram University (A Central University), Aizawl-796004, Mizoram, India, Tel: +91 9473583251; E-mail: sehrawat_r@yahoo.com
Abstract: Background: The aim of this study was to determination of Anti-Quorum Sensing (AQS) and anti-biofilm potential of the methanol extract of ginger (Zingiber officinale) rhizomes against multidrug-resistant clinical isolates of Pseudomonas aeruginosa (P. aeruginosa).
Methods: The AQS activity of ginger was determined against Chromobacterium violaceum (C. violaceum) ATCC 12472 (CV12472), a biosensor strain, in qualitative manner using the agar well diffusion method. The violacein pigment inhibition was assessed to confirm AQS activity of ginger. The AQS potential of sub-minimum Inhibitory Concentrations (sub-MICs) of the ginger extract was determined by targeting different QS regulated virulence factors, including swarming motility (using swarm diameter measurement method), pyocyanin pigment (using chloroform extraction method), Exopolysaccharide (EPS) (using phenol-sulphuric acid method), and biofilm formation (using microtiter plate assay), against clinical isolates (CIs 2, 3, and 4) and standard reference strain of P. aeruginosa (PA01).
Results: The AQS activity of methanol extract of ginger was confirmed against C. violaceum (CV12472) as inhibition of violacein pigment formation without effecting the growth of CIs and PA01 of P. aeruginosa. The ginger extract exhibited concentration-dependent inhibition of virulence factors and biofilm formation. The maximum reduction was found in swarming motility, pyocyanin, EPS and biofilm formation against PA01 (51.38%), CI3 (57.91%), PA01 (63.29%) and CI2 (64.37%), respectively at 1/2 MIC of ginger extract.
Conclusion: The results of present study revealed the effective AQS and anti-biofilm potential of Zingiber officinale rhizome methanol extract at a reduced dose (sub-MICs). The extract may be explored as an agent of antimicrobial compounds having AQS and anti-biofilm activity for controlling microbial infection and also for reducing the chances of emergence of resistance in P. aeruginosa.
Introduction :
Continuous increasing resistance against conventional antibiotics in pathogenic micro-organism has become a major obstacle in the treatment of microbial infections. In the present scenario, along with the new therapeutic agents, antimicrobial mechanism and alternate drug target should also be focused for the effective solution of Multidrug-Resistant (MDR) microbial infections. Pseudomonas aeruginosa (P. aeruginosa) is a gram-negative opportunistic bacterium that exhibits MDR pattern and a cause of nosocomial infection, urinary tract infection, burn wound infection, eye infection, respiratory infections and also infecting cystic fibrosis patients, Acquired Immunodeficiency Syndrome (AIDS), and pulmonary diseases 1-4. Antibiotics, are most commonly used for the treatment of infections caused by P. aeruginosa 5, generally depending on growth inhibition mechanism. Quorum Sensing (QS), a cell to cell communication gene regulatory mechanism, targets regulation of various virulence factors and biofilm formation rather than growth inhibition or killing bacteria in several gram-negative bacteria such as P. aeruginosa 6-8. In P. aeruginosa, the two major QS systems are lasI-lasR and rhlI-rhlR which are inter-related and functions in a hierarchical manner 9,10. lasI-lasR and rhlI-rhlR quorum sensing systems involve auto inducer signal molecule, N-(3-oxododecanoyl) homoserine lactone (OdDHL) and N-butanoyl homoserine lactone (BHL), respectively 11,12. Pseudomonas quinolone signal (PQS), (2-heptyl-hydroxy-4-quin-olone), a third intercellular signal has also been investigated in P. aeruginosa 13.
Various virulence factors such as swarming motility, Exopolysaccharide (EPS), pigments (pyoverdin, pyocyanin), alginate, LasA protease, LasB elastase and biofilm formation in P. aeruginosa are regulated by QS mechanism14,15. Bacterial biofilm is a group or communities of cells that is encased in self secreted extracellular polymeric substance consisting of proteins, polysaccharides and nucleic acids attached to a surface and involved in pathogenesis of infection and drug resistance 16. Biofilm formation in MDR P. aeruginosa may reduce the effect of antibiotics and enhance the chances of emergence of drug resistance 6. The treatment of infection with higher dose, overdose or prolonged uses of antibiotics is one of the major causes of drug resistance and biofilm formation. Comparatively the required doses of antibiotics or natural plant material for growth inhibition (antibacterial activity) are generally higher than required for interruption in QS cascade; therefore, regulation of virulence factors and biofilm formation by AQS mechanism at lower doses may be an effective solution of drug resistance and biofilm formation. The complications related to antibiotic therapy such as serious side effect in patients and chances of increasing drug resistance force the scientific community to investigate and explore the alternatives of antibiotics 17-19.
The medicinal plant extracts/phytoconstituents exhibit antibacterial and Anti-Quorum Sensing (AQS) activity may be explored as an effective alternative of antibiotic therapy. Ginger (Zingiber officinale Roscoe.) family Zingiberaceae, has been used in traditional system of medicine for management of many diseases. It has been widely used for the management of nausea, diabetes, fever, cough, loss of appetite, indigestion, abdominal pain, intestinal colic, inflammation, infertility insomnia, and urinary tract infections and also exhibit antibacterial, anticoagulation, antipyretic, anti-obesity, anti-tumorigenic, AQS and anti-biofilm activity 20-25. The major phytochemicals with diverse bioactivity including gingerol, shogaoal and paradol as phenolic compounds; zingiberene and bisabolene as volatile sesquiterpenes; and curcumene and citral as monoterpenoids 26, may be an effective alternative of synthetic compound or antibiotics against antibiotic resistant micro-organism 27,28. We have already determined the antibacterial activity and Minimum Inhibitory Concentrations (MIC) of methanol extract of ginger against reference PA01 and CIs 2,3,4 in our previous reported study 29. In the present research work the AQS and anti-biofilm potential of methanol extract of ginger were investigated at sub-MICs against multidrug-resistant clinical isolates of P. aeruginosa.
Materials and Methods :
Plant material collection and extract preparation: The fresh ginger (Zingiber officinale) rhizomes were collected and authenticated from Central Council for Research in Ayurvedic Sciences-Regional Ayurveda Research Institute, Jhansi (Uttar Pradesh), India. The air dried and powdered rhizomes were subjected for extraction with methanol (100%) 29. Briefly 10 gm of rhizome powder were soaked in methanol solvent and mixed for 3 successive days at room temperature. The methanol extract was filtered through Whatman filter paper and centrifuged at 3000 rpm for 10 min. Supernatant was concentrated with rotary evaporator. The methanol extract was aseptically stored in sterile air tight bottle at 4°C for further experiment.
Collection of clinical isolates and reference strains: Clinical isolates of P. aeruginosa were collected from Department of Microbiology, Sarojini Naidu Medical College, Agra (Uttar Pradesh), India. Reference strain (PA01) of P. aeruginosa and biosensor strain, Chromobacterium violaceum (C. violaceum) ATCC 12472 (CV12472) were collected from Department of Agricultural Microbiology, Aligarh Muslim University, Aligarh (Uttar Pradesh), India. P. aeruginosa were sub-cultured and maintained on Luria- Bertani (LB) broth at 37°C. C. violaceum (CV12472) was maintained on nutrient broth at 28°C.
Screening of antibacterial activity and determination of MIC: In the previous step of our research study, agar well diffusion method was used for screening of antibacterial activity of crude ginger methanolic extract against Clinical Isolates (CIs) and reference strain of P. aeruginosa and 96 well microdillution method was used to determine MICs 29 as per the guideline of Clinical and Laboratory Standard Institute 30.
Screening of preliminary AQS activity: The preliminary AQS activity of ginger extract was confirmed qualitatively as inhibition in quorum sensing controlled violacein pigment formation in biosensor CV12472 using standard agar well diffusion method 31 with slight modification. Mueller Hinton agar plate was prepared with 100 µl of freshly grown and aseptically diluted bacterial suspension (inoculum size of 2.5×106 CFU/ml). The wells of approximate 6-8 mm diameter were made sterile cub borer and bottom of wells were sealed using soft agar. 50 μl of ginger methanolic extract (500 mg/ml) was aseptically poured in wells and plate was incubated at 28°C for 18-20 hr. A well for negative control was made with DMSO (dimethyl sulfoxide). The diameter of inhibition zone around the well representing violacein pigment inhibition (mm) was measured to determine the AQS activity 32.
Determination of AQS and anti-biofilm activity against P. aeruginosa: Assay for swarming motility: The effect of ginger extract (at sub- MICs) on swarming motility was determined using the swarm plate method. The swarm plate was prepared using sterile 8 gm/l of nutrient broth added with 5 gm/l of glucose and 0.5% of bacto agar was aseptically mixed to solidify the media. The 50 μl of sub-MICs of ginger extract was aseptically added in the swarm agar. The agar was poured in sterile plates and plates were kept at room temperature to solidify 33. The swarm plates were point inoculated with freshly grown P. aeruginosa culture and incubated at 37°C for 18-24 hr. Swarm diameter (mm) (turbid zone form the point of inoculation) with treated and untreated P. aeruginosa culture (as control) was noted to calculate percentage inhibition in swarming motility 32.
Assay for pyocyanin formation: The chloroform extraction method 34 was used to determine the effect of ginger extract on pyocyanin pigment formation. Freshly grown bacterial culture (5 ml) were mixed with 150 µl of sub-MICs of ginger extract and incubated at 37°C for 18-24 hr. A total volume of 3 ml of chloroform were added to the culture supernatant and mixed vigorously. The chloroform layer was transferred in sterile tube and further 1 ml of 0.2 M HCl (hydrochloric acid) was added and solution was centrifuged (8,000 rpm for 10-12 min at room temperature). Absorbance of HCL layer (pink to deep red colour) in treated and untreated culture (as control) was noted at 520 nm to calculate percentage inhibition in pyocyanin formation 32.
Extraction and estimation of EPS: The bacterial isolates were grown in LB broth containing 150 μl of sub-MICs of ginger extract at 37°C for 18-24 hr and subjected for centrifugation after incubation. Supernatant was filtered and transferred aseptically in fresh sterile tubes. Three volume of 100% chilled ethanol was added and the tubes were incubated at 4°C for 24 hr to precipitate extraction and quantification of (EPS) 35. The phenol (5%)-sulphuric acid (concentrated) 36 was further used for quantification of EPS by measuring the sugar. Percentage inhibition in EPS was calculated by measuring the absorbance of treated and untreated culture (as control) at 490 nm 32.
Assay for biofilm formation: The anti-biofilm potential of ginger extract was determined using microtitre plate assay 37 with slight modification. Biofilm formation in the wells of microtitre plate were allowed in the presence of ginger extract (at different sub-MICs) at 37°C for 18-24 hr. After incubation, the wells were drained properly and washed three times with sterile Phosphate-Buffered Saline (PBS). The biofilm in the wells was stained with 150 µl of crystal violet solution (0.1%) and plate was incubated at room temperature for 5-10 min. After incubation, the wells were washed with sterile PBS to remove excess dye and plate was kept for air drying at room temperature. The dye taken by biofilm cells was solubilized with 150 µl of glacial acetic acid (33% v/v). Absorbance of treated and untreated culture (as control) was recorded at 570 nm to calculate percentage inhibition in biofilm formation 32.
Statistical analysis: The experiments were performed in triplicate and the mean±standard deviation was calculated. The data were further statistically analyzed with One way ANOVA and level of significance were expressed as P value (p˂0.001, p˂0.005, p˂ 0.01 and p˂0.05 were denoted with ****, ***, ** and * respectively) when inhibition in PA01 was compared to clinical isolates. Comparison between inhibitions by sub-MICs with in strain/clinical isolates was made and based on Tukey’s HSD post hoc test and the mean difference was expressed as significant at the 0.05 level. All the statistical analyses were performed in IBM SPSS statistics 20 (version 20.0. Armonk, NY: IBM Corporation).
Results :
The preliminary AQS potential of ginger extract was confirmed qualitatively as inhibition in violacein pigment formation using agar well diffusion method against C. violaceum CV12472 (Figure 1). As an extension of our previous reported study, the sub-MICs (1/2,1/4,1/8 MIC) of previously determined MIC of ginger extract were made (Table 1) and subjected to determine anti-quorum and anti-biofilm activity against CIs and reference PA01 of P. aeruginosa. The effect of ginger extract on growth of CIs and PA01 of P. aeruginosa at selected sub-MICs were determined using spectroscopic growth curve analysis and observed that growth of tested isolates were not affected by mentioned sub-MICs (data not shown). The concentration dependent reduction in swarming motility was observed in tested clinical isolates and PA01 by ginger extract. The maximum reduction in swarming motility was found against PA01 (51.38%) at 1/2 MIC. It was approximately similar against CI2, CI3 and CI4 at 1/2 MIC. The reduction in all tested isolates were slightly similar at 1/4 MIC (32.04-33.8%) and at 1/8 MIC (17.79-20.6 %) which showed that maximum reduction was possible at 1/2 MIC and approximately similar at the concentration of less than 1/2 MIC (Figure 2A). The ginger extract reduced the pyocyanin pigment formation against the tested isolates within the range (47.61-57.92%) at 1/2 MIC, (28.44-36.16%) at 1/4 MIC and (19.92-23.70%) at 1/8 MIC and maximum against CI3 (57.92%) (Figure 2B). The dose dependent reduction (%) in EPS was observed and the maximum reduction was found against PA01 (63.29%) followed by CI3 (43.66%) and CI2 (41.22%) (Figure 2C). The ginger showed maximum reduction in biofilm formation against CI2 (64.37%), followed by PA01 (60.36%), CI3 (58.89%) and CI4 (51.60%) at 1/2 MIC, while maximum reduction at 1/4 MIC was found against PA01 (46.28%) followed by CI2 (44.23%), CI4 (33.13%) and CI3 (28.61%) (Figure 2D).
Discussion :
Traditionally plant component/extract has been used for the treatment of microbial infections. From the past several decades’ growth inhibition mechanism is focused as one of the major targets for controlling microbial infections but in present scenario scientific community established that targeting QS mechanism through AQS approach may be a better solution for reduction in drug resistance and safe treatment of microbial infection at lower doses of antibiotics/plant component. In the present study, AQS and anti-biofilm potential of ginger was evaluated against MDR P. aeruginosa and observed the dose dependent reduction in the various virulence factors including swarming motility, pyocyanin pigment, exopolysaccharides and biofilm. Preliminary AQS activity of ginger extract was investigated and confirmed qualitatively as inhibition of violacein pigment formation against a biosensor strain, CV12472 (Figure 1) which showed similarity with previously reported findings with different plants including Eucalyptus globulus 32, Syzygium aromaticum 38,39, Terminalia bellerica 40, Xylopia aethiopica and Monodora myristica 41, Acacia nilotica, Cinnamomum verum and Punica grantum 39. The growth of P. aeruginosa was not affected by the tested sub-MICs of ginger extract which was in accordance of previous study reported Kim and Park in 2013. Pyocyanin pigment, associated in cystic fibrosis P. aeruginosa infections, is a well characterized QS regulated virulence factor. The concentration dependent reduction (%) in pyocyanin pigment formation was found with ginger extract against all tested CIs and reference PA01 (Figure 2B). Previously similar reduction in pyocyanin pigment formation with plant extracts was reported 40,42. The components of biofilm matrix keep the microbial cell together closely and serve as a barrier against antibacterial compounds to enhance its protection and drug resistance ability 42. The biofilm formation is a complex and multifactor dependent process in which EPS and swarming motility plays a key role for its formation in P. aeruginosa 43,44. Bacteria having ability of biofilm formation generally exhibit antibiotic resistance due to the formation of EPS as an essential component of biofilm maturation 45. Therefore, it was hypothesized that the regulation in biofilm formation may be achieved by controlling the EPS formation and swarming motility through interference in quorum sensing mechanism using plant extracts and consequently bacterial drug resistance may be regulated. Concentration dependent inhibition (%) in swarming motility was observed against CIs and reference strain PA01 (Figure 2A); similar reduction with plant extract has been previously reported 31,39.
In the present study concentration-based reduction (%) in EPS (Figure 2C) (for controlling biofilm formation) along with the reduction (%) in biofilm (Figure 2D) were found with ginger at sub-MICs in both the CIs and reference PA01. The effectiveness of ginger extract for reduction in biofilm formation (%) against P. aeruginosa reference strain was also reported 23,46. Yahya et al in 2013 reported the reduction in biofilm formation using different concentration of ginger ethanolic extract against P. aeruginosa 47. Miari et al in 2020 reported the anti-biofilm activity of ginger ethanol extract at 1, 3 and 5% and found 42.74% inhi-bition at 5%, while biofilm inhibitory activity was not observed at rest of any tested concentration below 5% (1% and 3%) against reference strain of P. aeruginosa which showed the minimum anti-biofilm inhibitory concentration (5%) without affecting growth 48. Similarly, we also observed the maximum biofilm inhibition (%) without affecting growth at concentration of 1/2 MIC (Table 1) and below which (at 1/4 MIC and 1/8 MIC) percentage reduction in biofilm was found comparatively low. Kim and Park in 2013 reported the 39-56% reduction in biofilm formation in P. aeruginosa (PA14) using different concentration of ginger extract (1, 5, and 10%). Reduction in EPS formation by 1% ginger extract in P. aeruginosa (PA14) has been also reported by Kim and Park in 2013. Akbari et al in 2023 reported the inhibition in biofilm formation with aqueous ethanolic extract of ginger against P. aeruginosa depending on the concentration of extract 49. Results of present study showed that the limit in reduction (%) of tested virulence factors and biofilm formation and required doses of ginger extract for observed reduction were isolates/strain dependent. The present study suggests that formation of biofilm may be regulated by controlling the EPS and swarming motility as both are the important factors for biofilm formation. Inhibition in different QS regulated virulence factors and biofilm formation was observed with plant extract in previously reported research studies 32,38,39,42, 50-52.
The present study revealed the potential of ginger for reduction of QS regulated virulence factors and biofilm formation (Figures 3A-3D), which may be an effective approach for controlling bacterial infection as well as drug resistance. Although AQS and anti-bio-film potential of ginger extract has been revealed through the present study yet molecular investigation is required to determine the actual site of action for QSI mechanism.
Conclusion :
The findings of present study suggest that the methanol extract of ginger rhizome, has an effective AQS and anti-biofilm activity at low dose (sub-MIC) for inhibition of various QS regulated virulence factors and biofilm formation; therefore, having potential for regulation in bacterial pathogenesis. Ginger may be explored as a weapon for the safe and effective treatment of infections caused by MDR P. aerugiona as well as controlling the chances of emergence of drug resistance.
Acknowledgement :
The authors thank to the Central Council for Research in Ayurvedic Sciences-Regional Ayurveda Research Institute, Jhansi (Uttar Pradesh), India for providing and authenticating the ginger (Zingiber officinale) rhizomes used in this research work. The authors gratefully thank the Department of Microbiology, Sarojini Naidu Medical College, Agra (Uttar Pradesh), India for providing the CIs of P. aeruginosa and the Department of Agricultural Microbiology, Aligarh Muslim University, Aligarh (Uttar Pradesh), India for providing Chromobacterium violaceum ATCC 12472 (CV12472) and the reference strain (PA01) for this study. For providing instrument/equipment facility for experiments, authors thank the Innovation centre, Bundelkhand University, Jhansi (Uttar Pradesh), India.
Funding: Nil.
Conflict of Interest :
There are no conflicts of interest.
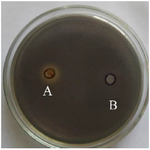
Figure 1. Inhibition in violacein pigment as antiquorum sensing activity of crude methanolic extract of ginger against C. violaceum CV12472. A) methanol extract of ginger; B) negative control.
|
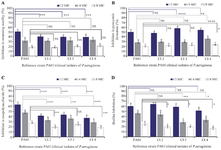
Figure 2. Inhibition of the formation of various virulence factors (A), Inhibition in swarming motility (B), Inhibition in pyocyanin formation (C) Inhibition in exopolysaccharide (EPS), and (D) Biofilm Inhibition. Each bar represents the mean value of three independent replicates, and the error bar shows the standard deviation. ****p<0.001, ***p<0.005, **p<0.01, *p<0.05, NS: not significant when the reference PA01 strain was compared to CI 2, 3, and 4. Values sharing a common letter (a, b, c) were not significant (at p<0.05) when comparison was made between inhibitions by sub-MICs with in strain/clinical isolates.
|
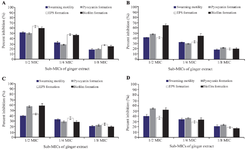
Figure 3. Inhibition (%) of quorum-sensing–regulated virulence factors and biofilm formation in the (A) reference strain PA01, (B) CI 2, (C) CI 3, and (D) CI 4 of P. aeruginosa by sub-MICs of the ginger extract. Each bar represents the mean value of three independent replicates, and the error bar shows the standard deviation.
|
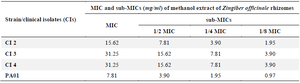
Table 1. MIC and sub-MICs of ginger extract against clinical isolates (CI) 2, 3, 4, and PA01 of P. aeruginosa
|
|