Production of Egg Yolk Antibody (IgY) against Vibrio cholerae O1: Protective Effect in Mice
-
Shoushtari, Mohammad
-
Anatomical Sciences Research Center Institute for Basic Sciences, Kashan University of Medical Sciences, Kashan, Iran
-
Asadi, Sepideh
-
Department of Microbiology and Immunology, Faculty of Veterinary Medicine, University of Tehran, Tehran, Iran
-
Karami, Yousof
-
Faculty of Veterinary Medicine, Shahid Bahonar University of Kerman, Kerman, Iran
-
 Zeinoddini, Mehdi
Faculty of Chemistry and Chemical Engineering, Malek Ashtar University of Technology, Tehran, Iran, Email: zeinoddini52@mut.ac.ir
Zeinoddini, Mehdi
Faculty of Chemistry and Chemical Engineering, Malek Ashtar University of Technology, Tehran, Iran, Email: zeinoddini52@mut.ac.ir
-
 Fathi, Javad
Department of Bacteriology and Virology, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran, E-mail addresses: javadfathi70@yahoo.com
Fathi, Javad
Department of Bacteriology and Virology, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran, E-mail addresses: javadfathi70@yahoo.com
Abstract: Background: Cholera is an acute intestinal infection caused by Vibrio cholera (V. cholera). The development of antibodies against specific V. cholerae may have a therapeutic effect. In the present research, we investigated the protective effect of egg yolk Immunoglobulin (IgY), which was produced by immunizing hens with formaldehyde-killed V. cholerae O1 and subsequently the isolated IgY was orally administrated to the V. cholerae O1 infected mice for evaluation of its immunizing capability.
Methods: In the current study, hens were immunized three times with formaldehyde-killed V. cholerae O1 (1.5× 107 CFU/mL) and an equal volume of adjuvant. The IgY was isolated from egg yolk by polyethylene glycol method. The validity and activity of isolated IgY were confirmed with SDS-PAGE and ELISA methods, respectively. Subsequently IgY was orally administered to suckling mice following challenge with V. cholerae O1. ELISA results showed high antibody titer in the serum and egg yolk. Also, SDS-PAGE analysis showed successful purification of IgY and anti-V. cholerae IgY prevented the death of mice infected with V. cholerae O1. The anti-V. cholera IgY was administered at 2, 4, 6 hours’ intervals after 3 hours of inoculation of mice with V. cholerae O1.
Results: Results showed that the rate of surviving mice (2 mg/mL of IgY) were 60% after 4 hours and 40% after 6 hours and the rate of surviving mice (5 mg/mL of IgY) was 70% after 4 hours and 60% after 6 hours.
Conclusion: The findings suggested the egg yolk-driven IgY as a natural antibacterial protein, could be effective in the prevention and treatment of cholera disease.
Introduction :
Vibrio cholera (V. cholerae) is a gram-negative, facultative anaerobic, curved and motile bacterium. Cholera is an intestinal infection caused by V. cholerae 1. Vibrio species have been isolated from seafood, seawater and vegetables 2,3. Cholera is often caused by two O1 and O139 serogroups 4. Endemic cholera is a vital health problem in developing countries, wherein every year, more than 100,000 people are infected and some of the patients lose their life. The highest occurrence of cholera is reported in Asian and African countries 5. Many cases of cholera are reported in travels. As the patient becomes dehydrated, the treatment includes Oral Rehydration Solution (ORS) 6. Although this therapy could be successful in many patients but it fails in some of them. Several vaccines have been used for cholera prevention. This vaccine includes an injectable vaccine and Oral Cholera Vaccine (OCV). Although vaccines may be effective, but the successful treatment requires large amount of vaccine during long period of time, which is not economically reasonable 7.
Several studies have shown that egg yolk-driven IgY is useful in controlling and inhibiting intestinal pathogens. IgY is the major low molecular weight immunoglobulin 8,9. Recent studies have shown that IgY is a passive, extensive and alternative antibody that could be easily produced. IgY is used as a natural antimicrobial protein for control and prevention of foodborne infection. It has enterotoxic activity. IgY is an effective antibody in prophylaxis. The advantages of using IgY compared to mammalian antibodies include non-invasive production, purification by a simple method, cost-effectiveness, and with no interaction with complement and mammalian Fc receptors 10. The use of IgY for prevention and control of intestinal infection has been demonstrated in a variety of pathogens including of Escherichia coli (E. coli) 11,12, Helicobacter pylori (H. pylori) 13, Rotavirus 14, Salmonella typhimurium (S. typhimurium) 15 and Staphylococcus aureus (S. aureus) 16. Here, we report the expression and isolation of egg yolk-driven IgY of immunized hens with formaldehyde-killed V. cholerae antigen. Subsequently the isolated IgY was orally administrated to the V. cholera infected mice to investigate its immunization effect.
Materials and Methods :
Preparation of cell antigen: In the present study V. cholerae (O1) was used. The V. cholerae (O1), (PTCC No: 1611) was obtained from the Iranian Research Organization for Science and Technology (IROST). The bacteria were initially cultured in Thiosulfate-Citrate-Bile Salts-Sucrose (TCBS) agar medium and incubated overnight at 37°C. Colonies were tested for biochemical characteristics through Grams staining, oxidase and catalase test. Also, the V. cholerae (O1) was cultured in Brain Heart infusion (BHI) broth (Merck Co. USA) for 15 hr at 37°C. After serial dilution and colony count, 1.5×107 colony-forming unit/ml (CFU/ml) was prepared. Bacteria were harvested by centrifugation at 3500 rpm at 4°C for 20 min. Then, washed twice with sterile Phosphate-Buffered Saline (PBS) and re-suspended. A 1.5×107 CFU/ml was inactivated by treatment with 1% formaldehyde for 15 hr or by heat at 60°C for 1 hr 17. Finally, prepared antigens were re-suspended in the same volume of PBS and stored at -20°C before the immunization of laying hens.
Immunization of hens with inactivated V. cholerae O1: Prior to the experiment, the hens were kept under care for three days in a standard animal room with a 16/8 hr light/dark cycle and a temperature of approximately 25°C. After examining the health of the chickens, they were used in the experiment. Inactivated V. cholerae O1 (1.5×107 CFU/ml) and an equal volume of freund's complete adjuvant (Sigma-Aldrich, USA) were mixed and injected (subcutaneously) into 25-week-old leghorn hens (purchased from industrial poultry farming,Tehran, Iran) test and control groups (2 laying hens/group) 13. Booster injections (freund's incomplete adjuvant), were given at 2-week intervals following the initial injection. PBS and complete adjuvant were injected into the control group. After each injection, blood was taken from the chickens and the serum (centrifuged at 3000 rpm for 10 min) was kept at -20°C. After the last injection, the immunization of hens was confirmed by the ELISA method. Eggs were collected weekly and stored at 4°C. The egg yolks were carefully separated from the white egg and freeze-dried, then kept at a temperature of -20°C until further use.
ELISA method: A 1.5×109 CFU/ml (V. cholerae) was coated on the 96-well plates and kept overnight at 4°C in carbonate buffer. Blocking was performed by using 100 μl of 5% (w/v) per well of skimmed milk in PBS (NaCl 0.8%, KCl 0.02%, Na2HPO4 0.29% and KH2PO4 0.02% in deionized water) followed by incubation at 37°C for 1 hr. The plate was washed with 20% PBS-T (Phosphate buffered saline with Tween-20) buffer 3 times. 100 μl of serum at 1:100 dilutions were added to the first well and followed by two-fold serial dilution and incubated at 37°C for 45 min. A 1/3000 solution of anti-IgY antibody conjugated with Horseradish Peroxide (HRP) enzyme (Sigma-Aldrich, USA) in PBS-T buffer was prepared and 100 μl of this solution was added to each well. The plate was washed and then 100 μl of substrate solution [0.8% o-Phenylenediamine (OPD) and 8% H2O2 in citrate buffer] was added to wells and kept in dark for 15 min. 100 μl of 2 M H2SO4 was added to stop the reaction. The absorbance was measured at 490 nm by ELISA reader (Perlong New Technology, China). The uncoated wells, as negative control blocked using 5% skimmed milk in PBS.
Isolation of IgY: Isolation of IgY from yolk was done by previous rapid and simple methods 18. Briefly, egg yolks were separated from the whites’ part. The egg yolks were mixed with two volumes of phosphate buffer (sodium phosphate 0.1 M, NaCl 10 mM, pH=7). 3.5% (w/v) poly ethylene glycol (PEG 6000) was added to the egg yolk and mixed with a magnetic stirrer at room temperature (RT) for 1 hr. The mixture was centrifuged at 800 rpm at 4°C for 20 min. The supernatant was collected and filtered through a Whatman no.1 filter paper to remove lipid traces. Purification of IgY was done by precipitation with 12% (w/v) PEG and mixed by magnetic stirrer at room temperature for 1 hr and mixed by magnetic stirrer at room temperature for 1 hr. The mixture was centrifuged in 8000 rpm at 4°C for 10 min. The pellets were dissolved in an equal volume of phosphate buffer and preserved at 4°C until further use. The IgY content was measured by the Bradford method. The obtained IgY antibodies were stored at -20°C.
SDS-PAGE analysis of IgY: 12% SDS-polyacrylamide gel electrophoresis was performed to analyze IgY 19-21. The samples (isolated IgY from non-immunized and immunized hens) were mixed with sample buffer (62.6 mM Tris-HCL, pH=6.8, 25% glycerol (v/v), 2% SDS (v/v) and 5% β-mercaptoethanol) and incubated 10 min at 95°C. 10 μl of the samples were loaded into each well. Pre-stained protein standard marker (Thermo Fisher Scientific Inc.) was used as a molecular weight marker. The IgY concentration in egg yolk was determined using the Bradford method.
Activity of IgY using ELISA: The reactivity of the IgY was analyzed using ELISA. The plate was coated overnight at 4°C by 1.5×109 CFU/ml (V. cholerae). After blocking with 5% (w/v) skimmed milk, 100 μl of a 1:100 dilution of IgY was added and the ELISA was done according to the described ELISA method 22,23.
Effect of IgY on cholera-infected mice: In this study, suckling mice (purchased from the Pasteur Institute, Iran) were challenged. The suckling mice were separated from their mothers and orally challenged with a 50 μl bacterial suspension. Vibrio strain was grown in LB culture medium, then harvested by centrifugation and suspended in sterile PBS. The inoculum contained bacteria determined by colony counting. Studies showed that V. cholera O1 caused diarrhea and death in 4-10 day-old suckling mice. All the suckling mice used in the experiments were 6 days old and weighed 5.3±0.1 g. 6-day-old suckling mice were infected with 1.5×107 and 1.5×108 CFU/ml (50 μl) of V. cholerae by oral administration with flexible needle. 4 and 6 hr after the inoculation, the mice were then administered with different amount of IgY (2 mg, 5 mg/ml) at different period of times. Survival of suckling mice was examined during a 48-hr period 24. Studies showed that V. cholera O1 causes diarrhea and death in 4-10 day-old suckling mice. 5-day-old suckling mice (purchased from the Pasteur Institute, Iran) were infected with 1.5×107 and 1.5×108 CFU/ml (50 μl) of V. cholerae by oral administration with a flexible needle. 4 and 6 hr after the inoculation, the mice were then administered with different amounts of IgY (2 mg, 5 mg/ml) at different periods of time.
Statistical analysis: All statistical analyses were done using the SPSS version 19 (SPSS Inc., Chicago, Illinois, USA). The values of experiments are the means of three independent experiments with indication of Standard Deviation (SD). p-values <0.05 were considered statistically significant.
Results :
Immunization of hens: After the immunization, hens produced eggs containing antigen-specific antibodies in their yolk. The ELISA results showed that antibody levels of the immunized hens were significantly higher than those of non-immunized hens (p<0.05). The result of ELISA is shown in figure 1.
Isolation of IgY: The egg-yolk proteins obtained (15 mg/egg) during the isolation of IgY were analyzed by SDS-PAGE 12% (Figure 2).
Activity of IgY using ELISA: The reactivity of IgY was determined by the ELISA method. Anti-whole cholera IgY well reacted with Vibrio bacteria (Figure 3).
Therapeutic effect of oral administration of IgY in mice: Suckling mice were infected with 1 and 10 LD50 of V. cholerae O1. 4 and 6 hr after the inoculation, suckling mice were tested with 50 μl of different amount (2 mg, 5 mg/ml) of anti- V. cholerae IgY. Anti-V. cholerae IgY completely prevented the death of mice infected with 1 LD50 V. cholerae O1. The results of 10 LD50 challenge of bacteria showed that the rate of surviving mice (2 mg/ml of IgY) was 60% after 4 hr and 40% after 6 hr and the rate of surviving mice (5 mg/ml of IgY) was 70% after 4 hr and 60% after 6 hr.
Discussion :
In recent years, immunoglobulin obtained from avian egg yolk is used for diagnosis and passive immunotherapy 25,26. Egg yolk-driven IgY is an inexpensive source of antibodies. The molecular weight of IgY is 180 kDa including the two heavy chains (H), each one with a molecular weight of 67 to 70 kDa, and two light chains (L), with 25 kDa. Also production of IgY in hens is easy with highly yielded antibodies 27,28. In many studies against Cholera Toxin B Subunit and Lipopolysaccharide (LPS) of V. cholerae, IgY were produced and their protective properties were evaluated 24,29,30; however, in the present study, whole and inactivated V. cholera cells were used for immunogenicity.
We preferred to study the whole bacterial cell rather than LPS to investigate the production of IgY against the V. cholerae. IgY is an immunoglobulin that can bind to LPS, and can also bind to other bacterial antigens. Additionally, IgY is a ligand that binds to a specific protein on the surface of bacterial cells. Investigating the whole bacterium can provide better insights into cellular and immune responses. Furthermore, LPS is a lipopolysaccharide located in the bacterial cell wall that stimulates the immune system. Therefore, investigating LPS alone may have effects on the immune system response that is not directly related to IgY 31-33. Hence, to study IgY in V. cholerae, we preferred to work on the entire bacterial cell in order to take advantage of all the antigens present in the bacteria. We have shown that preparation of IgY from the egg yolk of immunized hens with V. cholerae is effective in preventing the occurrence of V. cholerae in mice. The present research showed that 1 ml of egg yolk (15 mg/egg) contains about 9.4 mg of IgY, equal to 141 mg IgY per standard hen's egg. Accordingly, in a year, one hen lays about 250 eggs (about 4000 ml of egg yolk). Therefore in one year, about 40 g of IgY could be obtained from an immune hen 34. Many studies revealed that during the immunization of a hen, antibodies reside in the egg yolk and the level of antibody production would be very high 8,26. In this study, we extracted IgY with the PEG method. The result of injected antigen via the intramuscular route of hen showed a higher level of antibody after 28 days’ immunization. The anti IgY produced by the immunized hens was measured by ELISA. The specific IgY titer increased second week after the initial injection. Thus, a large amount of IgY could be easily prepared without reducing its activity. SDS-PAGE confirmed the extraction and isolation of IgY. The analysis of SDS-PAGE showed that IgY has two main bands as heavy and light chains. Many studies have shown, that IgY has anti-bacterial and anti-inflammatory effects. Moreover, oral administration of IgY-derived hen's egg yolk has been successfully used in the prevention of several diseases 11,13,14,16.
Earlier studies also indicated that UreC-induced IgY successfully inhibits the H. pylori infection 13. Another study reported the production of anti-Vibrio harveyi (V. harveyi) egg yolk immunoglobulin and evaluated its stability and neutralization efficacy 35. The results showed that IgY at concentrations of 1, 5 and 10 mg/ml efficiently inhibited V. harveyi within 24 hr after exposure.
In the present experiment, we used suckling mice to induce V. cholerae infection. Recent studies in several laboratories using animal models (suckling mice) have shown that immunization with antigen of V. cholerae can protect the animals against cholera disease 36. In this study, we demonstrated that IgY obtained from egg yolk of hens immunized with V. cholerae (O1) can inhibit the cholera bacterium. Our results show the affinity of IgY for the neutralization of V. cholerae. Higher survival of mice was observed in the test group pretreated with 2 mg/ml of IgY (60% after 4 hr) and 5 mg/ml of IgY (70% after 4 hr). Many studies have revealed that V. cholerae infection in suckling mice could be efficiently treated using oral administration of egg yolk-driven IgY from hens immunized with V. cholerae.
Conclusion :
The produced egg yolk-driven IgY could be implemented for passive immunotherapy and diagnosis of disease. IgY production is easy, affordable and high yield. Therefore, the findings suggest that IgY as a natural antibacterial protein, could be effective in prevention and treatment of cholera disease. Further clinical studies, under specific ethical considerations, on possible applications of this therapy in humans are suggested.
Ethics approval :
All procedures performed in studies involving animal participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments. All experiments and protocols were approved by the Ethics committee (working with laboratory animals) of Malek-Ashtar University of Technology, Tehran, Iran.
Acknowledgement :
The authors would like to thank the research council of Malek-Ashtar University of Technology (MUT), and Shiraz University of Medical Sciences (SUMS) for the financial support of this investigation. This research was carried out in project number IR-MUT 296391731103 under the supervision of research management of Malek-Ashtar University of Technology.
Funding: Not applicable.
Conflict of Interest :
Authors have no competing interests in this research.
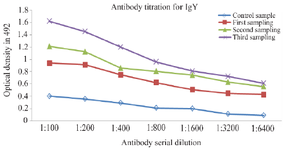
Figure 1. IgY titer in the serum of immunized and control chickens against V. cholerae O1.
|
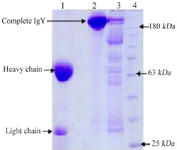
Figure 2. 12% SDS-polyacrylamide gel electrophoresis analysis of protein containing IgY from hen’s egg yolk. Lane 1: IgY from egg yolk of immunized hens (sample buffer containing 2-mercapto-ethanol). Lane 2: IgY from egg yolk of immunized hens (sample buffer without 2-mercaptoethanol). Lane 3: IgY from egg yolk of non-immunized hens, Lane 4: Protein marker.
|
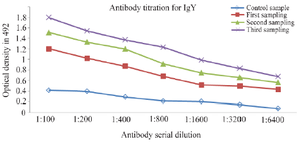
Figure 3. IgY titer in the egg yolk of immunized and control chickens against V. cholerae O1.
|
|