Characterization, Cytotoxicity and Anti-oxidant Studies of Phytoniosome Loaded with Ethanolic Leaf Extract of Tinospora Cordifolia
-
Masilamani, Sri Devi
-
Department of Biotechnology, Dr. MGR Educational & Research Institute, Maduravoyal, Chennai, India
-
Chokkalingam , Priya
-
Department of Biotechnology, Dr. MGR Educational & Research Institute, Maduravoyal, Chennai, India
-
 Hari, Rajeswary
Department of Biotechnology, Dr. MGR Educational & Research Institute, Maduravoyal, Chennai, India, Tel: +91 9840774705; E-mail: rajihar@gmail.com
Hari, Rajeswary
Department of Biotechnology, Dr. MGR Educational & Research Institute, Maduravoyal, Chennai, India, Tel: +91 9840774705; E-mail: rajihar@gmail.com
Abstract: Background: From time immemorial herbal preparations are been employed for the treatment of several ailments. In recent years due to poor bioavailability the conventional herbal preparations are replaced by phytoniosomes, an advanced novel drug delivery system in which the herbal extracts are incorporated into a non-ionic surfactant to yield higher absorption and remarkable desired pharmacological activity. The present study is aimed to prepare and characterize the ethanolic leaf extract of Tinospora cordifolia (nELETC) loaded phytoniosome and to compare its antioxidant properties with ethanolic leaf extract of Tinospora cordifolia (ELETC).
Methods: The ethanolic leaf extract and ethanolic leaf extract of Tinospora cordifolia loaded phytoniosome (ELETC and nELETC) were prepared. The characterization of the prepared phytoniosomes were performed by UV-Visible spectroscopy, FTIR, XRD, SEM, TEM, DLS and zeta potential. The nontoxic nature of the prepared phytoniosomes was analyzed using MTT assay in vero cell line. The antioxidant potential of ELETC and nELETC were compared by the scavenging activity of DPPH, Hydrogen peroxide and Superoxide radicals.
Results: The formation of ethanolic leaf extract of Tinospora cordifolia loaded phytoniosome (nELETC) was confirmed with UV-Vis spectroscopy. The SEM and TEM images confirmed the spherical shape of the nELETC with average size ranging from 600 to 1800 nm. The zeta potential showed magnitude of -65.55 to -77.83 mV and its crystalline structure was confirmed by XRD analysis. Through the FTIR spectrum presence of alcohols, alkanes, phenols, esters, aliphatic and aromatic compounds as well as alkenes and carbolic acids were identified. MTT assay establishes the non-toxic nature of the synthesized nELETC and excellent antioxidant potential was observed for nELETC than ELETC.
Conclusion: In conclusion, the ethanolic leaf extract of Tinospora cordifolia loaded phytoniosome (nELETC) will serve as a promising drug carrier in scavenging the free radicals and can be used in various biological applications.
Introduction :
Plant derived herbal drugs are used globally due to their easy availability, safe mode of action with less side effects and cost effectiveness. All over the world the primary health care system of underdeveloped and developing countries predominantly depends on the traditional drugs for the treatment of several dreadful diseases. The phytoconstituents present in the herbal drugs such as flavonoids, phenols, terpenoids and tannins contribute significantly towards the therapeutic activity of these plant drugs. Phytochemical and phytopharmacological sciences established the composition,
biological activities and health promoting benefits of numerous plant products 1. However, the bioavailability of these drugs is decreased due to their poor absorption which may be of either poor lipid solubility or larger molecular size 2.
The novel drug delivery system is the new approach for the plant derived drugs. A number of novel drug delivery system have emerged encompassing various routes of administration to achieve controlled and targeted drug delivery 3. In order to improve the therapeutic potential of the administered drug several types of drug targeting systems were developed. Drug targeting is defined as the distribution of drug in the body in such a way that the major part of the drug interacts entirely with the target tissue at cellular or subcellular level. The intention of drug targeting is to accomplish the desired pharmacological response at the selected site without any undesirable interactions at other sites 4,5.
Drug targeting of a standardized herbal drug is achieved by complexing them with phospholipid molecule containing phosphatidylcholine which improve the membrane permeability, water-oil partition coefficient and thus aid in the easy penetration through biological membranes and thereby improve the bioavailability of the drug 6. Nanocarriers encompassing the herbal constituents have a great capability to increase the therapeutic potential and to overcome the problems generally encountered with herbal medicines. In addition, they also help to carry optimum amount of the active constituents to the anticipated spot in the body by preventing the first pass metabolism and also aid their circulation in the blood 7. Nanocarriers in the form of niosomes are biocompatible, non-immunogenic, biodegradable molecules employed in recent years as an emerging drug delivery system. They have longer shelf life, enable delivery of drug at target site in a sustained manner and exhibit high stability. In recent years the phytoniosome technology is successfully applied to encapsulate herbal extracts as well as phytochemicals, with remarkable therapeutic potential 8.
Tinospora cordifolia is a medicinal plant which is categorized as "Rasayana" in ayurveda, considered as a tonic and a vitalizer. It is used to treat diabetes, heart disease, jaundice, allergies, skin problems, leprosy and urinary disorders 9. The whole plant possesses antiulcer, hepatoprotective, antioxidant, antidiabetic, cytotoxic and immunomodulatory activity 10,11. Powdered leaves and its decoction are proved to treat ulcers, jaundice, wounds, fevers and control blood glucose 12. It is also a rich source of alkaloids, sesquiterpenoids, lignans, sterols, polysaccharides, essential oils and fatty acids 13. The present study aimed to prepare and characterize phytoniosome loaded ethanolic leaf extract of Tinospora cordifolia.
Materials and Methods :
Collection and preparation of ethanolic leaf extract of Tinospora cordifolia (ELETC)
The leaves of Tinospora cordifolia were obtained from local market, Chennai, India and were authenticated by Dr. Mythreyi, Professor of Pharmacognosy department K.K. College of pharmacy, Chennai, India. The voucher specimen is deposited in above mentioned venue for future reference. The leaves were crushed into a coarse powder after shade drying and were extracted using 90% ethanol at room temperature by cold maceration process for three days. For solvent extraction the ethanolic extract was filtered and then evaporated using rota flash evaporator. The yield of the sample was calculated and the extract was preserved in the refrigerator till investigations. The yield was found to be 1.25% w/w.
Preparation of ethanolic leaf extract of Tinospora cordifolia loaded phytoniosome (nELETC): Tinospora cordifolia loaded phytoniosomes (nELETC) were synthesized through film hydration method 8. Initially, Tween 60 (10 mM) and cholesterol were dissolved in chloroform (1:1 M) and Tinospora cordifolia extract (1.0 mg/ml) was assorted in a round bottom flask. The excess of chloroform was removed (55°C) using the rotary evaporator to acquire skinny film of nELETC which was hydrated with phosphate buffer saline in a water bath at 55°C for 2 hr. The resultant solution was subsequently subjected to bath sonication (20 min) to obtain finer vesicles.
Entrapment efficiency of nELETC: The entrapment efficiency of the phytoniosomes loaded with Tinospora cordifolia extract was analyzed 14. The entrapment efficiency is the total amount of bio-active compounds present which was calculated as percentage of the bio-active compounds entrapped into purified phytoniosome. Dialysis was carried out to purify the phytoniosomes from the extract. The phytoniosomes present in the dialysis bag was dialyzed against distilled water overnight.
In order to rupture the niosomal membrane to release the free material, the phytoniosomes were allowed to treat with methanol. After purification, absorbance was read at 370 nm for the phytoniosome and the plant extract. The entrapment effectiveness was calculated from the given formula:
Entrapment efficiency = Amount of Phytoniosome in extractTotal amount of the plant extract ×100
Characterization of phytoniosomes: The Surface Plasmon Resonance (SPR) absorption bands had been measured using UV-visible Spectroscopy (Perkin-Elmer MA, USA), range between 350-750 nm. The possible functional groups in the synthesized phytoniosomes were analysed using Fourier Transform Infrared (FTIR Perkin-Elmer, MA, USA) spectra in the range of 4000-400 cm-1. The crystal structure of biosynthesized phytoniosomes were confirmed by X-Ray Diffraction pattern (XRD-Bruker AXS, Inc., Madison, USA). The size and shape of the phytoniosomes were measured using Scanning Electron Microscope (SEM) (Zeiss-German) and Transmission Electron Microscope (TEM) JEOL Japan, JEM-2100 Plus. The particle size and Polydispersity Index (PDI) of prepared phytoniosomes were measured using the Dynamic Light Scattering (DLS) method with a Zeta particle analyzer (Zeta-sizer Nano ZS-Malvern Instruments Ltd., UK) at 25°C.
MTT assay: Vero cells (African green monkey kidney normal cell line) were obtained from the National Centre for Cell Sciences (NCCS), Pune, India. Cells were maintained in the logarithmic phase of growth in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% (v/v) heat inactivated Fetal Bovine Serum (FBS), 100 U/ml penicillin, 100 μg/ml streptomycin. They were maintained at 37°C with 5% CO2 in 95% air humidified incubator. The cytotoxicity effect of the Tinospora cordifolia leaf extract loaded phytoniosome sample was tested against Vero cell lines by MTT (3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide assay 15. The cells were seeded in 96-well microplates (1×106 cells/well) and incubated at 37°C for 48 hr in 5% CO2 incubator and allowed to grow 70-80% confluence. Then the medium was replaced and the cells were treated with aliquots of nELETC extracts and incubated for 24 hr. The cells were then washed with Phosphate-Buffer Saline (PBS, pH=7.4) and 20 μl of (MTT) solution (5 mg/ml in PBS) was added to each well. The plates were kept in dark for 2 hr at 37°C. The formazan crystals were dissolved in 100 μl DMSO and the absorbance was read spectrophotometrically at 570 nm. Cell viability percentage was calculated using the formula,
Cell viability (%)=(Absorbance of sample/Absorbance of control)×100.
Sample–treated cells with nELETC extracts
Control–untreated cells
Graph was plotted using the cell viability (%) at Y-axis and different concentration of the sample in X-axis.
Anti-oxidant activity: DPPH radical scavenging assay: In the DPPH free radical scavenging assay, different concentrations (100-1000 µg/ml) of the test samples (ELETC and nELETC) in ethanol were mixed with 1 ml of freshly prepared DPPH solution and 2 ml of 0.1 M acetate buffer at pH=5.5 16. The resulting solutions were then incubated at 37°C for 30 min and measured spectrophotometrically at 517 nm. Standard antioxidant L-Ascorbic acid was used as positive control under the same assay conditions. Without extract or the inhibitor was taken for negative control. Higher DPPH scavenging activity was observed in lower absorbance at 517 nm. DPPH radical scavenging activity of the extract was calculated accordingly from the decrease in absorbance at 517 nm in comparison with the negative control with the given formula:
DPPH radical scavenging assay (%)=[(A0–A1/A0) ×100]
A0- Absorbance of the control
A1- Absorbance of plant extract and phytoniosomes.
Hydrogen peroxide radical scavenging assay: In this assay, hydrogen peroxide (40 mM) was prepared in pH=7.4 PBS. Various concentration ranges (200, 400, 600, 800, 1000 µg/ml) of the test samples (ELETC and nELETC) and ascorbic acid were separately added to hydrogen peroxide solution (0.6 ml). The reaction mixture was incubated for 10 min and observed spectrophotometrically at 230 nm 16.
Hydrogen peroxide radical scavenging assay (%)=[(A0 –A1/A0)×100]
A0- Absorbance of the control
A1- Absorbance of plant extract and phytoniosomes.
Superoxide scavenging activity: Superoxide radicals were generated in nicotinamide adenine dinucleotide, phenazine methosulphate (PMS-NADH) system by the oxidation of NADH and assayed by the reduction of Nitro Blue Tetrazolium (NBT). In this experiment, the superoxide radical was generated in 3 ml of sodium phosphate buffer containing 1ml of NBT solution, 1 ml of NADH solution, and different concentrations of ELETC and nELETC (100 to 1000 mg) in water. The reaction was started by adding 1 ml of PMS solution to the mixture. The reaction mixture was incubated at 25°C for 5 min and the absorbance was measured against the corresponding blank solution 17. L-Ascorbic acid was used as the positive control. The decrease of NBT reduction measured by the absorbance of the reaction mixture correlates with the superoxide radical scavenging activity of the extracts. The superoxide radical scavenging activity was calculated using the following formula:
Superoxide radical scavenging activity (%)=[(A0–A1/ A0)×100]
A0- Absorbance of the control.
A1- Absorbance of plant extract and phytoniosomes.
Statistical analysis: All the assays were done in triplicates and the statistical values were expressed in Mean±SE. By using the analysis of variance (ANOVA) followed by Dunnet’s "t" test the statistical significance is calculated. The p<0.05 were considered as significant.
Results and Discussion :
Entrapment efficiency of nELETC: In the present investigation the entrapment efficiency for the phytoniosome prepared from ethanolic leaf extract of Tinospora cordifolia was found to be 92.6% indicating the efficient entrapment. According to Hao Y-M, Li K 18, the affinity existing between the entrapped chemical and the vesicle material is the major contributor for the good entrapment efficiency. Since the entrapment efficiency of our nELETC phytoniosomes is also above 90%, we may expect a good interaction happening between the plant extract and the Tween 60 the vehicle material. This is because the polysorbate vehicles like Tween 60 are efficient carriers of hydrophilic and hydrophobic compounds as stated by Waddad et al 19.
UV-Vis analysis of Tinospora cordifolia loaded phytoniosome: UV spectroscopy is an excellent analytical tool used to confirm the niosome synthesis. The phytoniosomes synthesized from Tinospora cordifolia leaf extract was analyzed in UV visible Spectroscopy at 250-500 nm wave length. The peak for as prepared phytoniosomes was attained at 360 nm as shown in figure 1. This initial UV spectral examination aids in the validation of synthesis and stability phytoniosomes were generated from leaves of Tinospora cordifolia. Khalil RM et al 20 prepared lomefloxacin loaded niosomes for ocular preparation and observed the peak in the same 360 nm which further confirms the preparation of nELETC.
FTIR Analysis of Tinospora cordifolia loaded Phytoniosomes (nELETC): Figure 2 shows the FTIR analysis of niosomes prepared with Tinospora cordifolia projecting the presence of various biomolecules. The FTIR spectrum of prepared niosomes showed characteristic absorption peaks at 3441 cm−1 representing the presence of alcohols and phenols (O-H stretch, H-Bonded). The broad peak at 2921 cm−1 shows the presence of alkanes (C-H Strech). The sharp peaks at 1734 cm−1 and 1640 cm−1 represents the presence of esters, saturated aliphatic compounds and alkenes, respectively 21. A steady peak at 1459 cm−1 reveals the presence of aromatic groups (C-C Stretch). A sharp peak at 1249 cm−1 and 1100 cm−1 shows the presence of aliphatic amino compounds (C-N stretch). The medium peaks at 961, 845 and 521 cm−1 discloses the presence of carbolic acids, alkyl halides with O-H bend, C-Cl stretch and C-Br stretch, respectively. Garg et al 22 performed the phytochemical analysis of leaf extract of Tinospora cordifolia and reported the presence of various phytochemicals such as flavonoids, diterpenes, proteins, saponins, amino acids and sugars. The transmittance speaks observed with the prepared niosomes shows much relevance with most of the peaks corresponding to these chemical compounds. The same results were obtained by Miatmoko et al 23 in their studies representing phytosomes as nanocarriers.
SEM and TEM analysis of Tinospora cordifolia loaded Phytoniosomes (nELETC): In the present investigation figure 3 shows the Scanning Electron Microscope (SEM) images of the nELETC which represents the morphological nature of Tinospora cordifolia loaded phytoniosome. From the image it is observed that they have smooth spherical surfaces without pores and with average particle sizes of 834 nm. It was reported that the sizes of the niosomes could range widely, from about 20 nm to 50 μm 24. Our nELETC phytoniosomes size falls under this category. Phytoniosomes are termed to be self-assembled in nature and produced using some external forces such as heating or mechanical stirring energy as a driving force 25. The examination of the ultrastructure using TEM revealed that the prepared Tinospora cordifolia entrapped phytoniosomes were non-aggregated spherical shape particles (Figure 4), with sizes in agreement with those obtained by SEM.
Vesicular size and zeta-potential analysis of Tinospora cordifolia loaded phytoniosomes (nELETC): The vesicle size and surface charge represent critical function of niosomes. Vesicle formation and the size distribution of prepared niosomes has been recorded with diameter of 613 to 5203 nm (Figure 5) with relatively low PDI of 0.632±0.133 indicating the uniform consistency of the niosomes. The uniform distribution of the colloid (niosomes) in the suspension is maintained by its surface charge which is stabilized by electrostatic repulsive forces 26. The zeta potential and dispersity values of the niosomes directly influence the efficiency of the drug action. Vesicles with larger diameter are vulnerable for aggregations in various vital organs soon after its intake. Hence, it is important to maintain the appropriate zeta potential value range of niosomes 27. The electrostatic charge repulsion between the similar charge (either positive or negative) particles prevents the aggregation and thus ensure a dispersed state of the nanosuspension in the formulations 28. The surface charge of prepared niosomes was measured to be around -65.55 to -77.83 mV, thus representing the stability of the colloidal particles in the suspension for longer time.
XRD analysis of Tinospora cordifolia loaded phytoniosomes (nELETC): The crystalline structure of Tinospora cordifolia loaded phytoniosomes was investigated and it was shown in figure 6. The XRD pattern of the prepared phytoniosomes showed characteristic peaks at 26.97°, 28.03°, 30.34°, and 32.14°. The prominent peaks correspond to the crystallinity of drug loaded niosomes and the results can be related to the previous work 29. This also was in agreement with our results obtained under SEM that illustrated smooth surface characteristics of Tinospora cordifolia loaded phytoniosomes.
Cytotoxicity effect of Tinospora cordifolia loaded phytoniosomes (nELETC): MTT assay is an important technique in the study of toxicity, which is done for fast analysis of cellular metabolic activity upon cell exposure to various biological molecules. The cell viability was analysed for different concentrations of Tinospora cordifolia loaded phytoniosomes as shown in figure 7. After 24 hr, no toxicity effect was observed even at higher concentration which is in alignment with the results obtained by Madhuri et al 30, justifying the fact of entrapment and sustained release. Similar findings were reported using Marigold extract loaded niosomes 8.
Anti-oxidant activity: DPPH radical scavenging assay: The antioxidant activity of ELETC and nELETC extracts were assessed for DPPH radical scavenging activity (Figure 8). It shows the activity of these extracts in quenching the free radicals through the hydrogen donating capability 31. There was a dose dependent DPPH radical scavenging activity observed for the phytoniosomes, plant extracts and ascorbic acid. However, DPPH radical scavenging activity of the phytoniosomes exhibited potential inhibition when compared to the normal plant extract. This may be attributed to the fact that the entrapped phytochemicals present in the nELETC phytoniosomes has the ability to scavenge the DPPH radical efficiently.
Hydrogen peroxide radical scavenging assay: Figure 9 shows the hydrogen peroxide radical scavenging activity for ELETC and nELETC extracts. Hydrogen peroxide is a week oxidizing agent which can inactivate the enzymes, which is due to the oxidation of thiol groups 31. The percentage of inhibition of hydrogen peroxide radical increases with the concentration and is dose dependent. The phytoniosomes loaded extract showed more scavenging activity when compared to the plant extract.
Superoxide scavenging activity: Superoxide anions which are produced from incomplete metabolism of oxygen may directly or indirectly damage the biomolecules; thus, forming singlet oxygen. Thus, neutralization of superoxide radical is very much important to protect the cells from deleterious effect 32. The superoxide scavenging activity of phytoniosomes increased markedly with increase in concentration. This suggest that the phytoniosomes has a potent superoxide scavenging activity when compared to the plant extract (Figure 10).
Conclusion :
The present study was done for characterization of Tinospora cordifolia loaded phytoniosomes using UV spectroscopy, FTIR, SEM, TEM, XRD and zeta potential analysis. Subsequently the biological activity of the phytoniosomes was also examined by the cytotoxicity assay. Our findings indicate that the phytoniosomes showed no toxicity in vero cell lines. It can also be concluded that the phytoniosomes loaded extract exhibited high antioxidant and free radical scavenging activities, indicating Tinospora cordifolia loaded phytoniosomes is a significant source of natural antioxidant. This makes it helpful in prevention of various oxidative stress. Thus, Tinospora cordifolia loaded phytoniosomes has a promising potential for various biological applications.
Acknowledgement :
We gratefully acknowledge Er. A.C.S. Arun Kumar, President, Dr. M.G.R Educational and Research Institute University for providing the necessary facilities.
Conflict of Interest :
The authors declare that they have no conflict of interests in this paper.
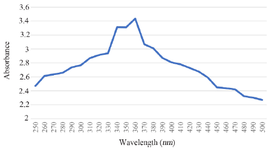
Figure 1. UV-Vis analysis of Tinospora cordifolia loaded phytoniosome.
|
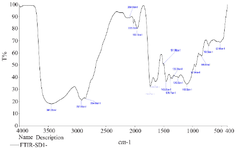
Figure 2. FTIR analysis of Tinospora cordifolia loaded phytoniosome.
|
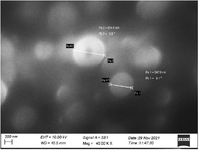
Figure 3. SEM image of Tinospora cordifolia loaded phytoniosomes.
|

Figure 4. TEM image of Tinospora cordifolia loaded phytoniosomes.
|
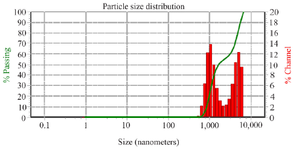
Figure 5. DLS analysis of Tinospora cordifolia loaded phytoniosomes.
|
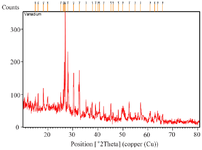
Figure 6. XRD analysis of Tinospora cordifolia loaded phytoniosomes.
|
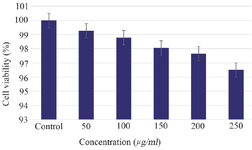
Figure 7. Cytotoxicity effect of Tinospora cordifolia loaded phytoniosomes. Values are mean±SE in each group. Statistical significant test for comparison was done by ANOVA followed by Dunnet’s "t" test.
p<0.05 is considered significant.
|
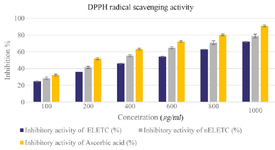
Figure 8. DPPH Radical Scavenging Assay. Values are mean±SE in each group. Statistical significant test for comparison was done by ANOVA followed by Dunnet’s "t" test.
p<0.05 is considered significant.
|
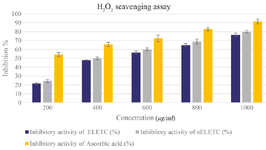
Figure 9. Hydrogen Peroxide Radical Scavenging Assay. Values are mean±SE in each group. Statistical significant test for comparison was done by ANOVA followed by Dunnet’s "t" test.
p<0.05 is considered significant.
|
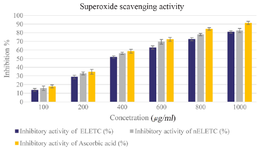
Figure 10. Super Oxide Scavenging Activity. Values are mean±SE in each group. Statistical significant test for comparison was done by ANOVA followed by Dunnet’s "t" test.
p<0.05 is considered significant.
|
|