Anti-proliferative potentials of Aconitum heterophyllum Root Extract in Human Breast cancer (MDA-MB-231) cell lines-Genetic and Antioxidant Enzyme Approach
-
Saravanan, Sujatha
-
Department of Biotechnology Dr M.G.R Educational and Research Institute Maduravoyal, Chennai, Tamil Nadu, India
-
 Hari, Rajeswary
Department of Biotechnology Dr M.G.R Educational and Research Institute Maduravoyal, Chennai, Tamil Nadu, India, Tel: +98 21 40774705; E-mail: rajihar@gmail.com
Hari, Rajeswary
Department of Biotechnology Dr M.G.R Educational and Research Institute Maduravoyal, Chennai, Tamil Nadu, India, Tel: +98 21 40774705; E-mail: rajihar@gmail.com
-
Sekar, Karthikeyan
-
Department of Biotechnology Dr M.G.R Educational and Research Institute Maduravoyal, Chennai, Tamil Nadu, India
Abstract: Background: One of the most important research activities around the world is the screening of various plant components for novel anticancer medicines. The anticancer activities of Aconitum heterophyllum were studied in human breast cancer MDA-MB- 231 cells in this study. Since tumorigenesis is thought to be the result of a series of pro- gressive gene alterations, including oncogene activation and tumour suppressor gene in- activation, the expression of genes like p53, p21, STAT, and Bcl-2, which are thought to be important in tumorigenesis and cell death, was determined. In the present study there was an upregulation in the level expression of p53and p21 and down regulation in the expression of BCL2 and STAT. However, there is increase and decrease level of gene expression in Aconitum heterophyllum roots loaded Phyto-Niosomes (nEEAH), when compared to ethanolic root extract of Aconitum heterophyllum EEAH extract treated MDA-MB-231 cell lines.
Methods: The enzymatic antioxidants such as CAT, SOD, GR, GST, and GPX as well as non-enzymatic antioxidants such as glutathione, Vitamin E and Vitamin C were esti- mated in the treated MDA-MB-231 cells at the end of incubation. The RT-PCR tech- nique was performed to study the expression patterns of apoptotic genes such as p53 and p21 and anti-apoptotic genes BCL2 and STAT in the drug treated MDA-MB-231 cells
Results: In the present study there was a significant (p<0.05) increase in CAT and glutathione levels and a decrease in Vit C, Vit E and SOD, GR, GST, GPX levels in the untreated MDA-MB-231 cells. Increased apoptotic gene expression and decreased anti-apoptotic gene expression suggest the anti-proliferative nature of the drug extract was comparable to the doxorubicin the positive drug used in the present study.
Conclusion: It can be concluded that the ethanolic extract of Aconitum heterophyllum roots loaded Phyto-Niosomes (nEEAH), when compared to ethanolic root extract of Aconitum heterophyllum EEAH extract treated MDA-MB-231 cell lines exert its anticancer activity by activating the apoptotic genes, suppressing anti-apoptotic genes as well as modulating the antioxidant enzymes.
Introduction :
Breast cancer is one of the most common cancers in women and is the second leading cause of cancer death after lung cancer among women worldwide 1. Though the etiology of breast cancer includes multiple factors such as environmental, genetic, social, demographic and hormonal, the role of oxidative stress in the occurrence of breast cancer cannot be ruled out 2. During the aerobic oxidative process of energy production by the metabolism of carbohydrates, proteins and lipids the highly Reactive Oxygen Species (ROS) such as superoxide, hydrogen peroxide and hydroxyl radicals are produced which are efficiently scavenged by the endogenous antioxidant defense systems in the form of enzymes namely Superoxide Dismutase (SOD), Glutathione Peroxidase (GPX), Catalase (CAT), Glutathione Reductase (GR), Glutathione S-transferease and non-enzymic antioxidant vitamin C, vitamin E, and glutathione 3,4. Oxidative stress is encountered by the cells whenever the critical balance between the production of ROS and their potential scavenging by these defense system is lost, leading to destruction of nuclear as well as mitochondrial DNA resulting in mutation, which in turn activate oncogenes and inactivate the tumor suppressor genes.
First described in 1979, and initially believed to be an oncogene, p53 was the first tumour suppressor gene to be identified. p53 functions to eliminate and inhibit the proliferation of abnormal cells, thereby preventing neoplastic development. Abrogation of the negative growth regulatory functions of p53 occurs in many, perhaps all, human tumours. The p53 signaling pathway is in "standby" mode under normal cellular conditions. Activation occurs in response to cellular stresses, and several independent pathways of p53 activation have been identified that appear to be dependent on distinct upstream regulatory kinases 5.
The Bcl-2 is the founding member of family of genes that either prevents or promotes cellular apoptosis 6,7. The Bcl-2 itself is an anti-apoptotic gene that prevents initiation steps of apoptosis and programmed cell death. Expression of the Bcl-2 gene has been shown to be regulated by estrogens in normal and cancerous mammary epithelial cells that are Estrogen Receptor (ER) positive. Expression of the Bcl-2 protein can be determined by immunohistochemical methods in ~80% of breast tumor samples with either node positive or negative 8,9.
One of the most prominent TF (Transcription factor) families in breast cancer is the Signal Transducers and Activators of Transcription (STAT) family, which is comprised of seven structurally similar and highly conserved members, namely, STAT1, STAT2, STAT3, STAT4, STAT5a, STAT5b and STAT6. STAT3 is activated through several cytokines, including Interleukin 6 (IL-6) and Interleukin 10 (IL-10), and growth factors, including Epidermal Growth Factor (EGF), Fibroblast Growth Factor (FGF) and Insulin-like Growth Factor (IGF) 10. Once these factors bind to their corresponding receptors, Janus kinases (JAKs) are activated 11. JAKs phosphorylate the cytoplasmic tail of the cognate receptor and STAT3 via its SH2 domain and binds to phosphorylated tyrosine residues. The phosphorylated STAT3 forms homodimers and translocate into nucleus and, thus, can exchange signals between the cytoplasm and nucleus. Upon translocation into the nucleus, pSTAT3 forms a complex with some coactivators, including p68, and binds to the promotor region of target genes to activate their transcription 12.
Natural products have played an important role throughout the world in treatment and preventing different human diseases including cancers 13,14. Vinca alkaloids and cytotoxic podophyllotoxins were discovered in the 1950s as first anti-cancer agents from plants 15. Novel anti-cancer agents have been developed in recent decades with the goal of increasing drug pharmacodynamics and bioavailability, as well as delivering targeted drug concentrations to cancer cells with minimal toxicity to healthy cells 16. Phytosomes represents advanced herbal drug technology that offers defined bioavailability of plant drugs over the herbal extract 17. Phytosomes has emarcated the undefined bioavailability of lipid insoluble secondary metabolites 18. In this concept, Aconitum heterophyllum, a member of the Ranunculaceae family, has long been used to treat various human diseases as anti-inflammatory and hepatoprotective agent. It is a good source of diterpene alkaloids, flavonoids. Aconitine alkaloids from Aconitum species play important role in antitumour activities which was studied earlier by the researchers 19. The main aim of this study was to evaluate the anti-cancer effects of Aconitum heterophyllum root extracts in human breast cancer MDA-MB-231 cells and to study the expression of p53, p21, STAT, and Bcl-2 genes.
Materials and Methods :
Preparation of Aconitum heterophyllum ethanolic root extract (EEAH): Aconitum heterophyllum roots (1 kg) were dried and grinded into a coarse powder at room temperature. The powder was sieved through a 40-mesh sieve and extracted in a soxhlet unit at 60°C with ethanol. After ethanol extraction, the filtrate was evaporated under decreased pressure using a rota flash evaporator till all the solvent was removed and the EEAH was then kept refrigerated for further studies.
Preparation of ethanolic extract of Aconitum heterophyllum loaded phyto-niosome (nEEAH): Aconitum heterophyllum roots loaded Phyto-Niosomes (nEEAH) were synthesized by film hydration method. Initially,10 mM of Tween 60 and cholesterol were dissolved in chloroform with 1:1 molar ratio and 1.0 mg/ml of Aconitum heterophyllum roots extract was mixed in a round bottom flask. The excess chloroform was removed at 55℃ using a rotary evaporator to obtain a thin film of nEEAH on the surface of the flask which was hydrated with Phosphate-Buffered Saline (PBS) by agitation in a water bath at 55℃ for 2 hr. The resulting solution was then subjected to bath sonication for 20 min to obtain finer vesicles. Phyto-niosomes were separated from the untrapped materials by performing dialysis against distilled water for overnight.
Cell culture maintenance: MDA-MB-231 (human breast cancer cell line) were obtained from the National Centre for Cell Sciences (NCCS), Pune, India. Cells were maintained in the logarithmic phase of growth in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% (v/v) heat inactivated Fetal Bovine Serum (FBS), 100 U/ml penicillin, 100 μg/ml streptomycin. They were maintained at 37°C with 5% CO2 in 95% air humidified incubator.
MDA-MB-231 cells were cultured in T-25 flasks and maintained in RPMI medium for 48 hr. The RPMI medium was supplemented with FBS and pen-strep. Once 70% confluence of cells was attained in the 6 well plate (approximately 4×105 cells), the cells were divided into four groups and the following treatments were given. Group-I control cells without any treatment, group-II cells were treated with EEAH 117 µg/ml, group-III cells were treated with nEEAH 73 µg/ml and Group-IV cells were treated with doxorubicin (10 µg/ml). The positive control was used in the present study. The treated cells were incubated at 37°C for 24 hr in a CO2 incubator.
Semi quantitative real time PCR analysis of p53, p21, STAT3 and BCL2 genes: The cells were collected and the total RNA was extracted from the cell pellets using TRIZOL method. One ml of TRIZOL reagent was added into cell pellet and homogenised by vortexing. The homogenised samples were incubated for 5 min at room temperature for complete dissociation of nucleoprotein complexes. After incubation, the samples were centrifuged at 12000 rpm for 10 min to remove cell debris. The supernatants were transferred in a fresh tube and 0.2 ml of chloroform was added to each tube. The samples were vortexed vigorously for 15 s and incubated for 3 min at room temperature. After incubation, the samples were centrifuged at 12000 rpm for 15 min at 4°C. After centrifugation, around 0.6 ml of the upper aqueous phase was transferred carefully without disturbing the interphase into fresh tube. 0.5 ml of isopropropanol was added into all the tubes and gently the tubes were inverted for mixing. The samples were incubated for further 10 min at room temp and centrifuged for 10000 rpm for 10 min. The precipitated pelleted RNAs were washed by adding 1 ml of 75% ethanol and centrifuged for 10 min at 8000 rpm. The washing step was repeated twice. The RNA pellets were air dried for 10 min and dissolved using 100 μl of RNase free water. RNA was quantified using a spectrophotometer and the purity of the sample was determined using A260/280 readings. Samples with A260/280 readings above 1.8 are free of any protein contamination. 100 ng of sample was run on RNA gel to check for DNA contamination after the samples were treated with DNase I from NEB [Catalogue#: M0303S] as per manufacturers protocol to eliminate any possible DNA contamination.
The total RNA present in the different experimental Human breast cancer (MDA-MB-231) cell lines were isolated using the total RNA isolation kit according to the manufacture instruction (TRIzol reagent (Invitrogen, USA). For the expression study purpose, a total reaction mixture of 10 ml volume contains 1 mg of cDNA template, 10 pmol/ml of each primer and a 2× solution of Prime Taq Premix (GeNet Bio, Nonsam, South Korea).
The reverse transcription of RNA was carried out using higher capacity cDNA reverse transcription kit (Applied Biosystems, CA, USA). The primer design for the genes p53, p21, STAT3 and BCL2 genes expression was done by referring NCBI/primer-BLAST tool software (Table 1). The Quantitect SYBR1 PCR kit (QIAGEN, Valencia, CA, USA) was employed for the amplification and the quantification of the resultant mRNA expression. The gene expression was recorded in terms of fold change using RT-PCR data generated from MxPro Software from Agilent Technologies comparing with normalization to β-actin values using the ΔΔCt comparative cycle threshold method. For data (using Light Cycler 96 SW 1.1 Software) analysis the experiments were performed in triplicates. Using the relative gene expression (ΔΔCt) method the PCR data were analyzed using the equation below:
Fold change=2-Δ(ΔCt)
Where, Ct (reference gene), ΔCt = Ct (target gene), Δ(ΔCt) = ΔCt (treated) – ΔCt (control).
Cell lysate preparation for antioxidant assay: Trypsinization was carried out in the treated cells using Trypsin-EDTA Solution, which was collected in the Eppendorf tubes and centrifuged for five min at 3000 rpm to collect the pellet. The collected pellet was resuspended in 200 µl of lysis buffer (0.1 M tris, 0.2 M EDTA, 2M NaCl, 0.5% Triton). The cell lysate prepared were incubated for 20 min at 4°C and was used for the analysis of antioxidant enzymes.
Estimation of enzymatic and non-enzymatic antioxidants: The following enzymatic antioxidants such as Catalase (CAT), Super Oxide Dismutase (SOD), Glutathione Reductase (GR), glutathione peroxidase and Glutathione-S-Transferase (GST) were analyzed in the cell lysate using established protocols. The non-enzymic antioxidants like GSH, vitamin C, vitamin E were also measured in the cell lysate. All the enzymatic and non-enzymatic parameters were estimated using Shimadzu spectrophotometer, UV-1601 model at specific wave lengths.
Results :
Enzymatic antioxidants: The levels of antioxidant enzymes like CAT, GR and GPx in control, EEEA and Doxorubcin treated MDAMB 231 cell lines are depicted in table 2. The enzymes such as SOD, GPx, GR and GST were significantly decreased (p<0.01) and CAT was increased in the untreated control cell lines when compared to the 73 µg/ml and 117 µg/ml nEEAH, EEAH and doxorubicin treated groups.
In the present study the cells treated with 73 g/ml nEEAH extract exhibited higher enzymatic antioxidants levels proving the fact that the phytochemicals present in the extract could scavenge the free radicals thereby reducing further complications of oxidative stress encountered by the cancer cells.
Non-enzymatic antioxidants: Table 3 depicts the levels of non-enzymatic antioxidants such as vitamin C, Vitamin A and Glutathione in different cell line groups. In accordance with the enzymatic antioxidants the levels of vitamin C and vitamin A were significantly (p<0.01, 0.05) decreased and glutathione levels were increased in untreated control MDAMB 231 cell lines. The nEEAH extract treatment restores the levels of these altered non-enzymatic antioxidants and it was more pronounced in the group II cells which were treated with the concentration of nEEAH 73 μg/ml of the extract.
P53 and P21 mRNA expression in nEEAH, EEAH extract treated MDA-MB-231 cell lines: In the untreated control MDA-MB-231 cells the mRNA expression of p53 and p21were found to be one-fold against nEEAH when compared to the EEAH extract treated cells in which the expression of p53 increased significantly to 1.04 and 1.58-fold, respectively for 117 μg/ml and 73 μg/ml of drug treatment. The similar increase in the fold change for the p21 was observed and it was found to be 1.09 and 2, respectively for the above said concentration of 117 μg/ml and 73 μg/ml EEAH, nEEAH extract treated MDA-MB-231 cell lines (Figure 1).
STAT3 and BCL2 mRNA expression in nEEAH, EEAH extract treated MDA-MB-231 cell lines: The expression pattern of STAT3 and BCL2 is shown in the figure 2. The mRNA expression of STAT3 and BCL2 significantly decreased in nEEAH, compared to EEAH extract treated MDA-MB-231 cell lines against the control untreated MDA-MB-231. Being oncogenes the 117 and 73 μg/ml of drug treated MDA-MB-231 cells showed 0.05- and 0.02-fold decrease, respectively against the control cells which usually show a fold change of 1. In the case of STAT3 mRNA expression the drug treatment showed a negative fold change of -1.02 and -3.02 for 117 μg/ml and 73 μg/ml concentration against the fold change of 1 in the case of control cells.
Discussion :
Reactive Oxygen Species (ROS) are produced in the mitochondria due to the metabolic reactions that occur in the respiratory chain and have varied biological features. At high concentrations of ROS, they induce apoptosis or necrosis thereby induces the cell death. At particular concentrations they act like an endogenous carcinogen to induce DNA mutation, inflammation, oncogenic stimulation, increased metabolic activity and mitochondrial damage leading to carcinogenesis 20. So, it is an accepted fact that cancer cells show increased oxidative stress. There are several antioxidant defence systems in terms of endogenous and exogenous antioxidants to combat the oxidative stress encountered by the cancer cells. The first line of endogenous antioxidant enzyme system comprises of enzymes such as CAT SOD, GPx, GR and GST. The exogenous antioxidants are molecules mainly supplied from external source either in the diet or as supplements which also plays important role in reducing the oxidative stress.
In our present investigation there is an increase in the level of CAT and decrease in the levels of SOD, GPx, GR and GST in the treated human breast cancer MDAMB 231 cell lines. Mates and Francisca 21 also reported the decreased level of SOD in the MCF-7 cell lines. SOD constitutes one of the main cellular antioxidant enzymes which catalysis the conversion of superoxide (O2-) to hydrogen peroxide (H2O2) which in turn gets converted into water with the help of CAT. The increased CAT in our present investigation may be due to the low levels of H2O2 because of the decreased activity of SOD. The same results were observed in studies by Kattan et al 22. Generally based on their concentration these ROS molecules either increase or inhibit the proliferation and promote apoptosis. In this present investigation the increased levels of superoxide radicals due to the decreased concentration SOD enzyme may have a proliferative effect in the MDAMB 231 cells. The treatment with our nEEAH, EEAH extract corrected the levels of the SOD and CAT; thereby scavenges the excess levels of ROS, leading the MDAMB 231 cells towards apoptosis. It is stated by Prasad S, et al 23 that ROS has the ability to inhibit the PTEN pathway, an important repairing check point in cell cycle progression leading to the continuous proliferation of cells irrespective of mutation or damaged cells.
Ethanolic extract of Aconitum heterophyllum contains alkaloids, steroids, saponins, glycoside and phenolic compounds 24. Several preclinical studies have proved that terpenoids may be a potential therapeutic agent in treating several types of cancer including breast cancer 25. These terpenoids have the ability to regulate various transcription and growth factors of intracellular signalling mechanisms; thereby inhibit the initiation and promotion of carcinogenesis as well as their invasion and metastasis, induce apoptosis and suppress tumor angiogenesis.
There is another parallel GSH-dependent antioxidant enzyme system namely Glutathione S-Transferase (GST), Glutathione Peroxidase (GPX) and Glutathione Reductase (GR) are involved in the detoxification mechanisms using GSH as co-substrate. GST mainly catalysis the transfer of electrophilic xenobiotics to reduced Glutathione (GSH), thereby convert it into oxidized glutathione (GSSG). The enzyme GPx in turn reduces hydroperoxides as well as H2O2 by utilizing GSH and converting it to GSSG. The oxidized glutathione (GSSH) produced due to the above-mentioned reactions, in turn regains its original reduced (GSH) state by GR, an NADPH-dependent oxidoreductase. In our present investigation control cells showed an increased concentration of GSH and decreased levels of GST and GPX. The increased GSH levels may be due to their underutilization by these enzymes. Freidelin (terpinoid) present in the plant is found to have antioxidant, free radical scavenging and hepatoprotective effect.
The p53 is a potent tumor suppressor transcription factor responsible for the regulation of cellular progression or its arrest in G1phase of cell cycle. The p21, usually named as cyclin-dependent kinase inhibitor, gets induced both by triggering of p53-dependent and p53-independent mechanisms 26. Once the DNA gets damaged the p53-p21 pathway gets activated in the cells, leading to the temporary arrest of G1 and G2 checkpoints of the cell cycle; thereby terminating DNA replication and cell division.
Transcription factor p53 plays a central role in the DNA damage response. Double-strand breaking is responsible for the activation of ATM, which in turn activates the CHK2 kinase, which is responsible for the phosphorylation of p53 at the N-terminal. Phosphorylation, in return, interferes in the binding of p53 to MDM2. p53 induces G1 arrest by transcriptionally targeting the p21 gene. p53 can also induce apoptosis by Bax and Puma, which are the proteins of apoptotic machinery. Independently also p21 is able to execute the G2 phase cell cycle arrest, by interacting with Proliferating Cell Nuclear Antigen (PCNA), an essential cofactor for DNA polymerases 27.
In the present investigation there is an upregulation of both p53 and p21 mRNA expression pattern in the Aconitum heterophyllum loaded phyto niosomes (nEEAH), when compared to the Ethanolic Extract of Aconitum Heterophylum (EEAH) drug treated cells indicating the tumor suppressive nature of the drug. According to Gulbis JM et al 28 antitumor activity of semisynthetic derivatives of Aconitum alkaloids bis[O-(14-benzoylaconine-8-yl)] suberate (BBAS) involved in cell cycle blockade and apoptosis induction can be modulated by p53 gene status. With the upregulation of the p53 gene due to semisynthetic derivative of our plant extract treatment in the breast cancer cell lines, it can be suggested that the Aconitum heterophyllum roots loaded phyto niosomes (nEEAH) may be administered as adjuvant in the treatment of cancer chemotherapy.
Though it was an accepted fact and earlier work by Bates S 29 which also confirms the dominant nature of p53-mediated apoptotic pathway, Chodoeva A et al 30 have shown that the alkaloids from the roots of Aconitum yesoense var. macroyesoense root extract treated A549 human lung carcinoma cells inhibit cell growth through G1 arrest. This could increase p53 transcriptional activation and DNA-binding activity or it may be due to the upregulation of p21 dependent G1 cell cycle arrest by Aconitum yesoense var. macroyesoense root extract in cancer cells. It can be stated that phyto constituents present in the root extract of Aconitum heterophyllum also could act in the above said manner to exhibit the cytotoxic activity on the actively proliferating cells. Moreover, the significant feature of nEEAH in triggering the p53-independent pathway of p21 cell cycle, makes its potential therapeutic usage in p53 mutated breast cancers.
In recent years, evidence has suggested the Bcl-2 family plays an important regulatory role in apoptosis, either as inhibitor (Bcl-2) or as activator (Bax) 31. Also, evidence indicates that antiapoptotic Bcl-2 proteins act to stabilize mitochondrial membrane integrity by preventing cytochrome c release and subsequent caspase activation 32. Bcl-2 named as B-cell CLL/lymphoma 2 family gene appears to be key player in the regulation of apoptotic process and decides the cellular fate 33,34. 11, 13:11, 16-Diepoxy-16, 17-dihydro-11, 12-secohetisan-2-ol 35 N-Deethyl-N formyllyaconitine 36 O-Methylaconitine 37 A. heterophyllum Methyl-N-succinoylanthranilate 38. These compounds either individually or in a synergistic manner may downregulate the antiapoptotic gene BCL-2 and trigger the MDA-MB-231 cells towards apoptosis.
The STAT family (signal transducer and activator of transcription) transcription proteins comprise a family of seven closely related transcriptional factors which get activated by cytokines, growth factors and other oncogenes to regulate a diverse array of cellular processes. Among them involvement STAT3 in 23 different types of tumors such as hematological malignancies, head and neck, lung, gastric, hepatocellular, colorectal, prostate, and breast cancers is documented by several researchers 39. Added to that, according to Yue and Turkson 40 STAT3 activation by genetic and pharmacological approaches have provided compelling evidence for STAT3’s critical role in cell proliferation, apoptosis, angiogenesis, immune response and metastasis in breast cancer. STAT3 is activated by phosphorylation of its tyrosine and serine residues via signaling from upstream regulators 41. This phosphorylation event induces dimerization between two STAT3 molecules via reciprocal phosphotyrosine–Srchomology domain 2 (SH2) interactions 42. Activated STAT3 dimers then translocate to the nucleus and bind to the consensus promoter sequence of their target genes to initiate transcription. Several new specific STAT3 inhibitors have been found in recent years. Structure optimization of these inhibitors for reduced cytotoxicity to normal tissues and higher stability may be an interesting direction for researchers 43. In our present investigation downregulation was observed for STAT 3 genes in the MDA-MB-231 breast cancer cell lines treated with the nEEAH extract. The phyto constituents present in the Aconitum heterophyllum root has the great potential of binding with STAT-3 genes as observed in this present study. Consequently, the transcriptional and translational products of STAT-3 genes will be inhibited leading to the downregulation of not only the STAT-3 pathway but also the cross talking other factors associated with STAT-3.
Results of the present study suggest that the phyto niosomes produced from the root extract of of Aconitum heterophyllum exhibited enhanced anti-carcinogenic activity and bioavailability compared to its EEAH, which may contain bioactive compounds, probably that prevents proliferation of MDA-MB-231 breast carcinoma cells by mechanisms such as induction of apoptosis. These data are the first report on the possible molecular mechanisms of action of Aconitum heterophyllum extracts on cancer cell proliferation. Additional studies are required to characterize the bioactive compounds responsible for the observed activities of Aconitum heterophyllum plants as a novel resource for new anticancer drugs.
Conclusion :
In the present study it may be concluded that there was a restoration of antioxidant levels in the ethanolic extract of Aconitum heterophyllum treated MDA-MB-231 cell lines. Added to that the extract played a significant role in the expression patterns, either upregulating or downregulating the apoptotic and antiapoptotic genes; thereby exhibited its potential role leading to the process of cell death in breast cancer cells. In our investigation the negative fold change expression exhibited by the STAT3 genes is an important finding, since it may serve as a pharmacodynamic target to minimize or inhibit the effects of STAT3 transcriptional factors and may be developed into a novel phyto therapeutic drug in the treatment of breast cancer in near future.
Acknowledgement :
We thank Er. A.C.S. Arun Kumar, President, Dr. M.G.R Educational and Research Institute University for providing the necessary facilities.
Funding: No funding was received for the preparation of this review.
Ethical Considerations :
Ethical issues have been observed by the authors.
Conflict of Interest :
The authors declared that there was no conflict of interest in the study.
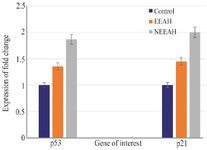
Figure 1. The expression pattern of the tumor suppressor genes p53 and p21 in nEEAH, EEAH extract treated MDA-MB-231 cell lines. Values are mean±SEM of three parallel measurements in each group Statistically significant test for comparison was done by ANOVA followed by Dunnet’s ‘t” test. Comparisons are made between:
a-Group I vs. Group II
b-Group I vs. Group III
*p<0.05, * *p<0.01, **p<0.001 NS–Not Significant.
|
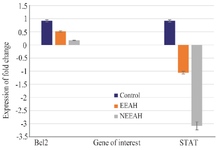
Figure 2. The expression pattern of the tumor suppressor genes BCL2 and STAT3 in nEEAH, EEAH extract treated MDA-MB-231 cell lines. Values are mean±SEM of three parallel measurements in each group Statistically significant test for comparison was done by ANOVA followed by Dunnet’s ‘t” test. Comparisons are made between:
a-Group I vs. Group II
b-Group I vs. Group III
*p<0.05, * *p<0.01, **p<0.001 NS–Not Significant
|
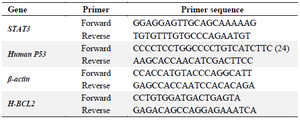
Table 1. The primer sequences used for p53, p21, STAT3 and BCL2 gene expression
|
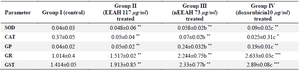
Table 2. Levels of enzymatic antioxidants in different experimental groups of human breast cancer MDAMB 231cell lines
Units: SOD-units/mg protein, CAT-μmoles of H2O2 decomposed/min/mg protein, GR-μmoles of NADPH oxidized/min/mg protein, GPx-μg of glutathione utilized/min/mg protein, GST-μmoles of CDNB conjugate formed/min/mg protein.
Values are mean SEM of three parallel measurements in each group Statistically significant test for comparison was done by ANOVA followed by Dunnet’s ‘t” test.
Comparisons are made between: a-Group I and Group II, b–Group I vs. Group III, c–Group 1 vs. Group IV.
*p<0.05, * *p<0.01, **p<0.001 NS–Not Significant.
|
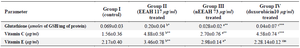
Table 3. Levels of non-enzymatic antioxidants in different experimental groups of human breast cancer MDAMB 231 cell lines
Values are the mean±SEM of three parallel measurements in each group. Statistical significance test for comparison was done by ANOVA followed by Dunnet’s ‘t” test.
Comparisons are made between: a-Group I and Group II, b–Group I vs. Group III, c–Group 1 vs. Group IV.
*p<0.05, * *p<0.01, **p<0.001 NS–Not Significant.
|
|