Immunogenic Potency of Formalin and Heat Inactivated E. coli O157:H7 in Mouse Model Administered by Different Routes
-
Arshadi, Nasim
-
Department of Biology, Faculty of Basic Sciences, Shahed University, Tehran, Iran
-
 Mousavi , Seyed Latif
Department of Biology, Faculty of Basic Sciences, Shahed University, Tehran, Iran, Tel: +98 21 51212232; E-mail:slmousavi@shahed.ac.ir
Mousavi , Seyed Latif
Department of Biology, Faculty of Basic Sciences, Shahed University, Tehran, Iran, Tel: +98 21 51212232; E-mail:slmousavi@shahed.ac.ir
-
Amani , Jafar
-
Applied Microbiology Research Center, Systems Biology and Poisonings Institute, Baqiyatallah University of Medical Sciences, Tehran, Iran
Abstract: Background: Enterohemorrhagic Escherichia coli (E. coli) (EHEC) O157:H7 is a major foodborne pathogen causing severe disease in humans worldwide. Cattle are important reservoirs of E. coli O157:H7 and developing a specific immunity in animals would be invaluable. The administration of Whole Cell Vaccines (WCV) is a well-established method of vaccination against bacterial infections. Route of administration, inactivation and using suitable adjuvant have significant effects on the characteristics and efficacy of WCV.
Methods: In the present study, an attempt was made to evaluate the immunogenic potency of heat and formalin inactivated cells administered orally and subcutaneously in mouse model by ELISA. Mice pretreated with streptomycin were used as a model to evaluate the efficacy of subcutaneous versus oral administration of the vaccine. Following immunization, mice were infected with E. coli O157:H7 and feces were monitored for shedding.
Results: Both forms of inactivated cells induced immune response and hence protection against infectious diseases caused by E. coli O157:H7. However, formalin inactivated cells of E. coli O157:H7 showed superior antigenicity compared to heat inactivated cells. Subcutaneous immunization of mice with both heat and formalin inactivated E. coli O157:H7 induced significant specific levels of IgG antibodies but did not lead to significant antigen-specific IgA rise in feces, whereas oral immunization elicited significant levels of IgG antibodies with some animals developing antigen-specific IgA in feces.
Conclusion: Inactivated E. coli O157:H7 is highly immunogenic and can induce protective immune responses via oral immunization.
Introduction :
Enterohemorrhagic Escherichia coli (E. coli) EHEC O157:H7 is a major food and water-borne pathogen which causes diarrhea, bloody diarrhea, hemorrhagic colitis and thrombotic thrombocytopenic purpura in humans, especially in young children and the elderly 1. Hemolytic Uremic Syndrome (HUS) is the most serious sequela of E. coli O157:H7 infection that occurs on average in 4% of infected humans 2. A number of factors have been identified to contribute in E. coli O157:H7 colonization of gastrointestinal epithelium, including fimbriae/pili, autotransporters, outer membrane proteins, flagella and Type III Secretion System (T3SS) 3. Intestinal colonization of pathogenic bacteria and release of Shiga toxins are important factors in infection of EHEC 1. Cattle are the primary animal reservoir of the gastrointestinal pathogen which can be directly acquired from beef/dairy products or indirectly via fecal shedding into the environment leading to contamination of other products or water supplies 3. Because of this, majority of EHEC control studies are focused on the eradication of this bacterium from the gastrointestinal tract of ruminants, whether by improved breeding practices or by vaccination 4.
Currently, there are few effective interventions to reduce the risk of this infection. Antibiotics are still effective treatment for O157 infection, while their usage promotes release of EHEC Shiga toxins, which increases the chance of complicating HUS 5.
The management of HUS requires control of bleeding, anemia, fluid and electrolyte imbalances, and other sequelae 6. Thus, vaccination remains one of the most promising pathways against E. coli O157:H7 infection.
Reducing E. coli O157:H7 in the cattle could decrease the risk of infection in human. For this purpose, several vaccines have been developed in animal models which include recombinant proteins like Stx1/2, intimin, EspA, fusion proteins of A and B Stx subunits, a virulent ghost cells of EHEC O157:H7, live attenuated bacteria expressing recombinant proteins, recombinant fimbrial proteins and DNA vaccines 6.
The administration of Whole Cell Vaccines (WCV) is one of the well-established methods of vaccination against bacterial infections. The main advantages of WCV include the presentation of many antigens particularly the protective ones. Moreover, minimal chances of side effects when given non-parenterally, zero virulence potential, and adjuvant-like character can be enumerated as other favorable features. Inactivated vaccines have been prepared by a variety of methods. Formalin and heat inactivation are the most commonly utilized methods for WCV 7.
The aim of this study was to evaluate the efficacy of inactivated bacteria as a vaccine. Since in the WCV, antigens are provided in the natural form with known and unknown immunogens together, they produce a strong and enduring immune response. But recombinant subunit vaccines have some limitations, such as booster shots to get ongoing protection against diseases. Vaccination with formalin or heat inactivated bacteria administered orally or subcutaneously to block colonization of E. coli O157:H7 on small intestine has been compared.
Materials and Methods :
Bacterial strains and culture conditions: Standard reference strains of E. coli O157:H7 ATCC: 35218 stored at -80°C in Luria-Bertani (LB) broth containing 20% glycerol, were grown on LB broth at 37°C with aeration of 150 rpm up to the late exponential phase.
Strain characterization: The gene coding for rfbE was amplified from genomic DNA extracted from E. coli O l57:H7 for strain confirmation. Primers used for amplification of rfbE gene were gifted by Dr. S. Nazarian (Imam Hussein University, Tehran, Iran).
PCR reaction mixture contained 3 mM of MgCl2, 0.4 mM of each dNTP, 1×PCR buffer, 1 U of Taq DNA polymerase (Fermentas), 1 µl of DNA template and 0.4 pm of each primer. Temperature conditions included initial denaturation at 94°C/4 min, denaturation at 94°C/40 s, hybridization at 57°C/1 min, and polymerization at 72°C/1 min for 32 PCR cycles. The primers used for confirmation of bacterial strain are detailed in table 1.
Preparation of formalin and heat inactivated bacteria: E. coli O157:H7 was cultured in LB at 37°C for 14 hr. For immunogenic studies, bacteria were grown to OD600: 0.6 and collected and washed thrice in sterile Phosphate-Buffered Saline (PBS, 137 mM NaCl, 2.7 mM KCl, and 4.3 mM Na2HPO4, pH=7.2) by centrifugation at 8000×g for 15 min at 4°C.
Cells were mixed with 0.4% formalin (MERCK, Germany) in PBS (pH=7.2) at 37°C for 1 hr in shaker, and then incubated for 18 hr at 4°C. Formalin-killed cells were collected at 10000×g for 30 min at 4°C and the cell pellet was washed with sterile PBS, resuspended in PBS to a final volume of 108 and 109 Colony Forming Units (CFU). The sterility test of formalin inactivated cells was performed onto Sorbitol MacConkey agar (SMA) (Que lab, Canada), and incubation was done for 7 days at 37°C 8-11.
To prepare heat killed E. coli O157:H7, bacteria were suspended in PBS and centrifuged at 8,000 g for 15 min. The resulting pellet was washed thrice and resuspended in PBS with concentration of 108 and 109 CFU. Cell suspensions were heated at 70°C for 1 hr in normal pressure. In order to confirm complete killing, aliquots of the resulting cell suspensions were spread onto SMA plates and incubated at 37°C for one week 12.
Animals: Female five-seven week-old BALB/c mice were obtained from Razi Institute (Karaj, Iran). Mice were housed in accordance with standard laboratory conditions with access to food and water ad libitum, in an environmentally controlled room with 12 hr light and dark cycles. A total of 30 mice were used to determine immunological response and rate of shedding post-challenge upon oral/subcutaneous administration of formalin and heat killed E. coli O157.
Vaccination: Thirty female BALB/c mice weighing 20-25 g were randomly divided into six groups for two different experiments. In the first experiment, mice groups 1 and 2 subcutaneously received formalin and heat inactivated E. coli O157:H7, respectively. The third group was given PBS as a control. The immunization program is summarized in table 2.
Inactivated bacteria for subcutaneous immunization were suspended in PBS and emulsified with Alum as an adjuvant (Razi Institute, Karaj) with the ratio of 50:50 (volume/volume). The final amount of antigen for each subcutaneous immunization was equal to 108 bacteria. Subcutaneous immunizations were done four times (1, 14, 28, 42 days). In the second experiment, 109 bacteria were administered by oral gavage to mice 4 times (1, 7, 14, 21 days). Prior to oral immunization, 100 µl of sodium bicarbonate (10%) was administered by oral gavage to neutralize the acidic content of stomach 9. The immunization program is summarized in table 2.
Serum collection: Blood samples were collected from all mice groups via retro-orbital plexus. The blood samples were incubated at 37°C for 1 hr and then incubated 1-2 hr at 4°C. Sera were collected by cold centrifugation for 15 min at 5000×g. The serum was collected and stored at -20°C until further use 2,13.
Fecal immunoglobulin extraction: Seven days after the last oral vaccination, fresh fecal pellets were collected and added to the extraction buffer (0.01 M ice-cold PBS, pH=7.2, 0.5% Tween, and 0.05% sodium azide) at a ratio of 1 ml per 100 mg fecal pellets wet weight. Tubes were vortexed for 15 min at room temperature and the fecal suspensions were centrifuged at 3000×g for 15 min at 4°C. A portion of the supernatant (400 µl) was transferred to a sterile eppendorf tube containing100 µl glycerol to which 10 µl of Phenyl Methyl Sulphonyl Fluoride (PMSF, Sigma) solution was added and then vortexed briefly. Samples were centrifuged at 14,000×g for 15 min at 4°C. Supernatants were transferred to sterile tubes and stored at -20°C until use 14.
Enzyme-linked immunosorbent assay (ELISA) for estimation of antibody titers: Analyses of serum IgG and IgA and mucosal IgA were carried out by indirect ELISA. Each well was added with 108 appropriated bacteria in 100 µl of the coating buffer with pH=9.6 and incubated overnight at 4°C 14,15. The binding of residual protein was blocked with 100 µl of 1% BSA in PBST at 37°C for 1 hr. A series of negative controls were included concurrently with removing all ELISA components. Serially diluted serum samples (1:100 to 1:25, 600) and fecal pellet extracts (1:1 to 1:128) were added to wells and incubated 2 hr at 37°C. The wells were washed three times with PBST, and horseradish peroxidase goat anti-mouse IgG (Razi, 1:15000) or horseradish peroxidase goat anti-mouse IgA (sigma, 1:1200) was added to the wells. The plates were incubated at 4°C for 1.5 hr, then added with freshly prepared TMB substrate solution (Mono bind, USA) and incubated for 10 min at room temperature in the dark. The reaction was stopped by the addition of 3N H2SO4, and the absorbance was measured at 450 nm using a microplate reader (Sunrise remote, Tecan-Austria) to quantitate the amount of bound antibody. All samples were run in triplicate 12,16-18.
Challenging the immunized mice: Mice were challenged 10 days after last immunization. Prior to challenges, mice were given drinking water containing streptomycin sulfate (5 g/L) to reduce the normal bacterial flora of gut. Following one day of treatment with streptomycin, mice were fasted overnight, and subsequently animals were inoculated by intragastric administration of approximately 1010 CFU of E. coli O157:H7. The extent of bacterial colonization was monitored every day for three weeks by quantitation of the E. coli O157:H7 shed into fecal pellets. E. coli O157:H7 fecal shedding was monitored by approximately 0.1 g of feces in 1 ml of LB broth followed by incubation at 37°C for 1-2 hr to allow the fecal pellets to soften. The mixture was then vortexed until the pellets were no longer visible. Serial dilutions of the supernatant were plated onto Sorbitol MacConkey agar plates containing tellurite. Plates were incubated overnight at 37°C and E. coli O157:H7colonies were enumerated. Bacterial colonies were tested for the O157 antigen by rfbE PCR 2,17.
Statistical analysis: Data were analyzed using GraphPad Prism 6.01 (GraphPad Software Inc., California, USA). Before calculations, all values were log 10 transformed and mean value±SEM (Standard error of the mean) was calculated. Multigroup comparisons were performed using one way ANOVA. When the test was significant, further pairwise comparisons were performed using Tukey’s test. p-values of less than 0.05 were considered statistically significant.
Results :
Strain characterization: The properties of strain used in this study were confirmed by PCR and biochemical tests. The rfbE gene of E. coli O157 encoding the O157 LPS is unique to the E. coli O157 serogroup (Figure 1) 19.
Antibody responses to immunization: Antibody titers of mice sera and fecal pellets after immunization with the inactivated E. coli O157:H7 were estimated. The mice were able to produce high serum IgG antibody titers after both subcutaneous and oral immunizations. Oral immunization resulted in significantly increased specific IgA titers (p<0.05) against E. coli O157:H7 in the feces and serum, compared to control mice. The serum IgG, IgA and fecal IgA ELISA results are summarized in figures 2 and 3.
IgG antibody response in serum: Both oral and subcutaneous immunizations resulted in increased IgG antibody titers to E. coli O157:H7 compared to that of control mice (p<0.05) (Figure 2A and B).
IgA antibody responses in serum and fecal extracts: IgA antibody responses to E. coli O157:H7 antigens in feces and serum were also studied. Oral immunization resulted in significantly increased specific IgA titers (p<0.05) against E. coli O157:H7 in the feces and serum compared to control mice (Figure 3A and B). Both the frequencies and magnitudes of IgA responses were lower than the IgG responses.
Challenging of immunized mice with E. coli O157:H7: In order to determine whether E. coli O157:H7 antigens in immunized mice could reduce or prevent the E. coli O157:H7 shedding in feces, subcutaneously and orally immunized and non-immunized mice were orally infected with 1010 CFU of E. coli O157:H7.
Shedding of the animals was monitored in feces. Shedding results are summarized in figures 4A and 4B. Non-immunized control mice shed high levels of E. coli O157:H7 (104-108 CFU) in their feces over the three weeks sampling period whereas shedding of nearly all immunized mice decreased gradually and stopped completely after 7-18 days (Figure 4). EHEC colonization in animals’ intestines was completely stopped after 8-10 days following immunization with formalin or heat inactivated bacteria through oral immunization route (Some animals immunized with heat inactivated bacteria subcutaneously continued shedding till day 18th). Significant differences were observed in the colonization of immunized and non-immunized mice but no significant differences were observed between immunized mice (orally and subcutaneously) using Tukey's multiple comparison test (p=0.050).
Discussion :
E. coli O157:H7 is the major pathogenic serotype of EHEC, a zoonotic enteric pathogen associated with sporadic outbreaks and illness 17. The prevalence of EHEC infections in humans has become a global public health problem 16, because exposure to antibiotics not only prevents pathogenesis but can even promote release of E. coli O157:H7 Shiga toxin by stimulation of the lytic cycle through bacterial SOS response 20. Therefore, controlling and eradicating E. coli O157:H7 infection is a challenging scientific problem and needs to be solved. Elimination of infectious agents, cutting off the transmission and protection of the vulnerable populations are three main elements in controlling communicable diseases. Vaccination is the most economic and effective means to reduce the incidence and protect humans and cattle against E. coli O157:H7 infection. Several vaccination approaches have been evaluated for EHEC. Among the vaccine candidates, those with immunological importance include Shiga toxins, virulence factors such as Tir, intimin, EspA and flagellin 21. These vaccines are mostly confined to few protein based antigens of T3SS and there might be other unknown antigens and excluding them reduces the efficacy of the vaccine. On the other hand, for many enteric pathogenic bacteria, due to the presence of polysaccharide on cell surface, protein based vaccines may not provide adequate protection. Specific polysaccharide of E. coli O157:H7 conjugated to endotoxin A of Staphylococcus aeruginosa (S. aeruginosa) was the first conjugate vaccine candidate 22. Although 6 months after the first injection, the antibody titers for IgG-LPS was 20 folds higher compared to pre injection with no significant adverse reaction, conjugate vaccines are chemically complex and their design, manufacturing and characterization is cumbersome. WCV can provide all potentially known and unknown antigens and overcome aforementioned problems and thereby offer an economically and potentially safe, effective means of preventing disease.
Differences in vaccine strains, conditions of growth, methods of inactivation, number of bacteria per dose, animal model and dosing schedules may explain the differences in efficacy of WCV 23. In this study, the effects of 4 different vaccine formulations were evaluated with 2 control groups. Different combinations of formalin/heat inactivated whole cells of E. coli O157:H7 were administered subcutaneously or orally. All vaccine formulations resulted in a significant reduction in total bacterial shedding (Figure 4).
The inactivation method can have significant effects on the characteristics of the vaccine 7. Both the heat and formalin inactivated vaccine formulations are shown to have complete protective capacity 24.
However, formalin inactivation of E. coli O157:H7 antigenicity showed better response compared with heat inactivated cells administered orally. This is due to the fact that surface antigens could retain their conformations on the formalin inactivated bacteria, but not on heat inactivated one. Animals immunized subcutaneously with heat killed E. coli O157:H7 containing 108 CFU showed significant increase of IgG antibody titers in comparison with the control group 5. Vibrio cholera (V. cholera) inactivated with phenol and heat was found to be a comparatively poor immunogen. The replacement of phenol with formalin greatly increased the antibody titers. The antibody produced against the formalin-inactivation was capable of recognizing surface antigenic determinants of heterologous strains of V. cholera. Antibodies produced against pathogenic bacteria cannot identify normal flora bacteria, because the antigenic structure of normal flora bacteria and their attaching factors differ from pathogenic bacteria. In this vaccination, antibodies are being developed only against the attaching factors of pathogens 23,25. Heat Killed Multi-serotype Shigella (HKMS) immunogens were evaluated in a guinea pig and broad spectrum protection in a guinea pig against shigellosis was determined 12. Formalin-killed whole-cell preparation of five main diarrheagenic E. coli pathotypes, formulated as a combined vaccine candidate, offered protection against the five main diarrheagenic E. coli pathotypes in a single vaccine using mouse model 9.
Aside from the choice of immunogen, the route of administration is also important for developing a successful vaccine. In our study, vaccines were administered orally and subcutaneously.
For subcutaneous immunization, only high serum IgG antibody titers were obtained, whereas oral immunization resulted in not only high titers of serum IgG antibody but also high titers of IgA antibody in mice serum and feces (Figure 3A and B). Fan et al evaluated the efficacy of subcutaneous versus intranasal administration of the recombinant Tir as vaccine. Only high serum IgG antibody titers were raised with subcutaneous immunization, whereas intranasal immunization resulted in both high titers of serum IgG antibody as well as IgA antibody in mice serum and feces. Mucosal immunization stimulates immune responses and produces sIgA prior to bacterial colonization 16. Subcutaneous immunization of mice with type III secreted proteins induced significant EspA and Tir specific serum IgG antibodies but did not significantly induce any antigen-specific IgA in feces, whereas intranasal immunization elicited significant EspA and Tir specific serum IgG antibodies with some animals developing antigen-specific IgA in feces 26. Safe and tolerable oral WCV may be the most effective and practical way of preventing enteric disease and other mucosal diseases as well 7.
Shedding below 104 CFU/g feces did not occur in the control group, indicating that vaccination may have a significant impact on E. coli O157:H7 carriage in mice through reducing mice-to-mice transmissions, even though vaccination may have little impact on the rate of clearance of bacterial infection.
In summary, the inactivated bacteria E. coli O157:H7 is highly immunogenic and can induce protective immune responses via oral immunization.
Conclusion :
WCV should continue to receive more attention as potentially effective, safe, and economical prophylactic and therapeutic treatments for infectious disease. Safe and tolerable oral WCV may be the most effective and practical way of preventing enteric and other mucosal diseases as well. The inactivation procedure may directly affect the functionality of the WCV, and selection of the inactivation method for WCV preparation should receive significant consideration.
Acknowledgement :
This work was supported by the Deputy of Research, Shahed University, Tehran, Iran.
Conflict of Interest :
The authors declare that they have no conflict of interest.
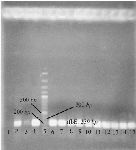
Figure 1. Agarose gel electrophoresis of PCR product of E. coli O157:H7 in order to confirm the bacteria. Lane 1: negative control; lane 2,4: positive control; lane 3: other bacteria; lane 5: 100 bp DNA ladder; lane 6-15: PCR product of the rfbE gene of E. coli O157:H7 grown in SMA.
|

Figure 2. Specific serum IgG following oral versus subcutaneous immunization with inactivated bacteria. There were significant differences in antibody titer between immunized and control mice groups (p˂0.05). A) IgG titer in the whole cell recipient group immunized subcutaneously, B) IgG titer in the whole cell recipient group immunized orally (Data represents the mean value±standard error of the three readings).
|

Figure 3. E .coli O157:H7 specific serum and fecal IgA following oral immunization (A) Serum IgA titer whole cell and (B) Fecal IgA titer whole cell in different mice groups 7 days after the final immunization (Data represents the mean value±standard error of the three readings).
|

Figure 4. E. coli O157:H7 shedding in feces following subcutaneous (A) and oral (B) administration in mice. Immunized and non-immunized mice were orally fed with 1010 E. coli O157:H7 and shedding was monitored in the feces for 21 days. Differences were considered significant whenever p<0.05. The limit of detection for plating was 1010 CFU/0.1 g feces. XY graphs are used for presenting data.
|

Table 1. Primer sequences used in this experiment
|
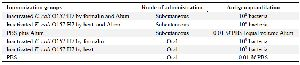
Table 2. Immunized groups, route of administration and antigen quantitation
|
|